Physiology News Magazine
Making old muscles young again – a therapeutic role for ββ2-agonists?
James Ryall and co-workers ask whether β2 anabolic enhancers might help prevent muscle loss in the elderly
Features
Making old muscles young again – a therapeutic role for ββ2-agonists?
James Ryall and co-workers ask whether β2 anabolic enhancers might help prevent muscle loss in the elderly
Features
James G Ryall, David R Plant, & Gordon S Lynch
Department of Physiology, The University of Melbourne, Victoria 3010, Australia
https://doi.org/10.36866/pn.56.33

What is sarcopenia?
Some of the most serious consequences of ageing are its effects on skeletal muscle. ‘Sarcopenia’ (derived from the Greek words ‘diminishing flesh’) is the term widely used to describe the slow but progressive loss of muscle mass with advancing age. Sarcopenia is characterised by a loss of both muscle quantity and quality that leads to a gradual decline in strength and a slowing of movement. The loss of strength is so dramatic it can remove an elderly person’s ability to carry out the tasks of daily living and rob a person of their functional independence, whilst also increasing the risk of sudden falls and fractures. As the number and proportion of older persons in the population continues to escalate, sarcopenia will have a dramatic impact on the quality of lives, and place ever-increasing demands on public health systems.
The loss of muscle mass and strength is thought to be due to the progressive atrophy and loss of muscle fibres associated with a loss of motor units, and a reduction in muscle ‘quality’ due to the infiltration of fat and other non-contractile material such as connective tissue. In some instances, there is a preferential loss of fast muscle fibres, leaving muscles with a greater proportion of slow fibres which, along with alterations in intracellular calcium handling, contributes to the slowing of movement. Motor unit remodelling (whereby denervated fast fibres subsequently become reinnervated by slow motoneurons) can also account for these changes. These age-related changes in skeletal muscle can be attributed to a complex interaction of many factors that affect neuromuscular transmission, muscle architecture, fibre composition, excitation-contraction (E-C) coupling, and metabolism (Plant & Lynch, 2002).
The goal of any strategy for treating sarcopenia, or any condition where muscle wasting is indicated, is to preserve and, ideally, increase muscle size and strength (Lynch, 2002a). One way to slow the progression of sarcopenia is through regular exercise, particularly training programmes involving resistance exercise or strength training (Singh, 2002). Although resistance training is clearly the best exercise to improve functional strength, and there are many health benefits associated with this form of training that can improve quality of life in the elderly, it must be recognised that exercise alone will not prevent sarcopenia. Also, there is a general reluctance to exercise, particularly in the elderly, and this further highlights the need for other therapeutic approaches. Elite Master’s level athletes who train and compete year in and year out for a large portion of their lifespan, simply do not perform at the same level when old that they did when they were younger. Clearly, there are other factors that contribute to the preservation of muscle quantity and quality.
Other approaches that are complementary to exercise also need to be considered. Given the magnitude of the growing public health problem related to sarcopenia, there is enormous interest in the development and evaluation of therapeutic strategies for preventing or ultimately reversing age-related muscle wasting and weakness.
Hormone replacement therapy for treating sarcopenia?
Ageing is associated with a so-called ‘somatopause’, defined as the decrease in circulating levels of anabolic hormones, including (but not limited to) growth hormone (GH), insulin, insulin-like growth factor I (IGF-I) and testosterone. These hormonal changes are thought to be responsible, at least in part, for the age-related loss of muscle mass and strength. Numerous clinical trials on older adults have focused on increasing circulating levels of hormones back to adult levels, as a way to combat sarcopenia. However, recent evidence from the US Women’s Health Initiative (combined Hormone Replacement Therapy) trial indicated that, for postmenopausal women, the risks for side effects (such as heart disease) from combined oestrogen and progestin appear to outweigh the benefits. Therefore, at this stage, it appears that this approach has only limited therapeutic applicability. In the absence of successful hormone replacement therapies, other muscle anabolic agents have been proposed to treat sarcopenia.
Role for β2-adrenoceptor agonists
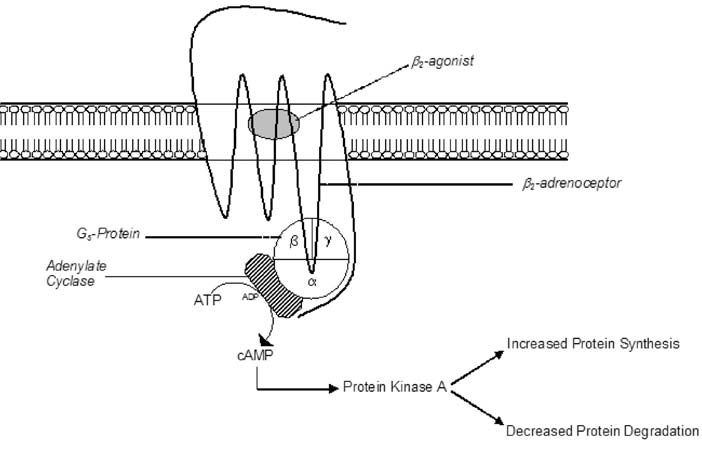
Although traditionally prescribed for alleviating bronchospasm in the treatment of asthma, β2-adrenoceptor agonists (β2-agonists, such as the most widely described, clenbuterol), when given systemically at higher doses, have potent anabolic effects on skeletal muscle. β2-agonists cause muscle hypertrophy via a cyclic AMP-dependent mechanism that results in an increase in protein synthesis and a decrease in protein degradation (Fig. 1). This hypertrophic effect, combined with a known lipolytic action, has proved desirable for those working in the livestock industry trying to improve meat quality and yield. Unsurprisingly, β2-agonists have also been used/abused by those engaged in competitive bodybuilding and soon after by other athletes competing in strength- and power-related sports.
As a therapy for treating muscle wasting and weakness β2-agonist administration has produced promising, although not entirely conclusive, results in many animal-based studies and some studies involving human patients (for review, see Lynch 2002b). When given to both young and old rats, the clenbuterol-induced increase in muscle mass was equivalent regardless of age, supporting the hypothesis that β2-agonists could be an effective intervention for countering sarcopenia (Carter et al. 1991). However, treating aged rats with a micromolar dose of clenbuterol, comparable to that used to treat asthma, did not prevent the age-related loss of muscle mass and strength (Chen & Alway, 2000). A more powerful β2-agonist, and/or at a higher dose, may therefore be necessary for treating sarcopenia effectively.
We have recently shown that, at an equimolar dose to clenbuterol, another β2-agonist, fenoterol, has a 10-15% greater anabolic effect on rat fast-twitch (EDL) and slow-twitch (soleus) muscles (Ryall et al. 2002). In a follow-up study we found that after just 4 weeks of daily administration, fenoterol completely ameliorated the age-related loss of muscle mass and strength in aged (28 month old) rats. The fenoterol-induced increase in mass and strength was attributed to hypertrophy of existing fibres and not to an increase in fibre number. To our knowledge, this was the first study to demonstrate complete restoration of both muscle mass and strength to (adult) control levels following β2 -agonist administration (Ryall et al. 2004).
One advantage of treating sarcopenia using β2-agonists rather than other anabolic agents relates to the ability of some β2-agonists to cause a shift in muscle fibre type proportions, typically from slow- to fast-twitch. Thus, powerful β2-agonists, such as fenoterol, are able to not only prevent, or reverse, the loss of muscle mass and strength, but might also help retain a higher proportion of fast-twitch fibres that will attenuate (in part) the characteristic slowing of contraction in aged mammals.
Too good to be true?
Despite the positive attributes of β2-agonists for treating sarcopenia, they have several deleterious side-effects, especially when administered in high doses. Given that β2-agonists act via the β2-adrenoceptor, and there exists a population of β2-adrenoceptors in the heart, it is difficult (perhaps impossible) to separate the hypertrophic effect on skeletal muscle from that on the heart. Cardiac hypertrophy has been observed in nearly all studies that have examined the effects of β2-agonist administration on skeletal muscle. Most of these studies have employed high doses in order to produce skeletal muscle hypertrophy and therefore, not surprisingly, they have also resulted in significant (and potentially deleterious) increases in heart size and, in some cases, fibrosis. This has so far limited the clinical potential of β2-agonists for sarcopenia, and also for other muscle wasting disorders (such as muscular dystrophy and sepsis). It is also important to note that many athletes are not aware of these potentially deleterious effects of chronic high-dose β2-agonist administration. For developing an effective therapy for sarcopenia, one must prevent or reduce these detrimental effects on tissues other than skeletal muscle, and the challenge is to devise treatments that utilise different β2-agonists that are capable of eliciting skeletal muscle hypertrophy at extremely low doses, and following only a short treatment duration.
Recently, new generation β2-agonists have been approved by the Food and Drug Authority (FDA) for the treatment of asthma. These β2-agonists, specifically salmeterol and formoterol, have been developed specifically to have an increased duration of action for the treatment of asthma, and a greater β2-adrenoceptor selectivity (the predominant skeletal muscle subtype) compared with existing β2-agonists. These new β2-agonists have the potential to elicit skeletal muscle hypertrophy at very low (micromolar) doses, due to their extended duration of action, whilst at the same time minimising any unwanted side-effects, due to the greater level of selectivity. Their efficacy and safety for application to muscle wasting disorders remains untested.
To sum up, there is a considerable need for therapies that can slow the effects of aging on muscle function. Strategies are needed to restore muscle size and strength in the frail elderly so that quality of life can be maintained or improved. Physical activity must continue to play an important role in all these therapeutic approaches; however, it must be realised that exercise alone will not prevent sarcopenia.
Consideration must therefore be given to other strategies including muscle anabolic agents. Although β2−agonists show considerable potential as an intervention for sarcopenia, much research is needed to test their efficacy and safety, especially the need to separate skeletal muscle and cardiac effects. These issues need to be addressed before they can be recommended for clinical application.
Acknowledgements
Supported by research grants from the Muscular Dystrophy Association (USA), Pfizer Pharmaceuticals (USA), and the Rebecca L Cooper Medical Research Foundation (Australia). JGR is supported by a Postgraduate Biomedical Scholarship from the National Heart Foundation (Australia).
References
Carter WJ, Dang AQ, Faas FH & Lynch ME (1991). Effects of clenbuterol on skeletal muscle mass, body composition, and recovery from surgical stress in senescent rats. Metab Clin Exp 40, 855-860.
Chen KD & Alway SE (2000). A physiological level of clenbuterol does not prevent atrophy or loss of force in skeletal muscle of old rats. J Appl Physiol 89, 606-612.
Lynch GS (2002a). Novel therapies for sarcopenia: ameliorating age-related changes in skeletal muscle. Expert Opin Ther Pat 12, 11-27.
Lynch GS (2002b) Beta-2 Agonists. In Performance-Enhancing Substances in Sport and Exercise, ed. Bahrke M & Yesalis C, pp 47-64. Human Kinetics, Champaign Illinois.
Plant DR & Lynch GS (2002). Excitation-contraction coupling and sarcoplasmic reticulum function in mechanically skinned fibres from fast skeletal muscles of aged mice. J Physiol 543, 169-176.
Ryall JG, Gregorevic P, Plant DR, Sillence MN & Lynch GS (2002). β2-agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am J Physiol 283, R1386-1394.
Ryall JG, Plant DR, Gregorevic P, Sillence MN & Lynch GS (2004). β2-Agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol 555, 175-188.
Singh MA (2002). Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. J Gerontol 57, M262-M282.