
Physiology News Magazine
MAMMALIAN TWO-PORE DOMAIN POTASSIUM CHANNELS
The two-pore domain potassium channels are now a new and important family with characteristics of leak potassium channels, crucial to regulation of cell excitability. Here their properties are explored by Alistair Mathie and Brian Robertson.
Features
MAMMALIAN TWO-PORE DOMAIN POTASSIUM CHANNELS
The two-pore domain potassium channels are now a new and important family with characteristics of leak potassium channels, crucial to regulation of cell excitability. Here their properties are explored by Alistair Mathie and Brian Robertson.
Features
Alistair Mathie and Brian Robertson
Neuronal Excitability Group, Biophysics Section, Blackett Laboratory, Imperial College of Science, Technology and Medicine, Prince Consort Road SW7 2BW
https://doi.org/10.36866/pn.43.5
Over the last two or three years, a new superfamily of potassium (K) channels, the two pore domain K channel family (2P-K), has emerged to join the established voltage-gated (Kv) and inward rectifier (K1R) potassium channel families (Gray, 2000; Lesage & Lazdunski, 2000; Goldstein et al., 2001 ). Uniquely, the individual subunits of this novel family have 2 pore regions (see Figure l ), that is to say, two separate regions of the subunit contribute to the single pore region of the functional channel. It is envisaged that two subunits come together to form functional dimers (rather than the usual K channel tetramers) although there is little direct evidence that this is indeed the case. In mammals, each subunit consists of four putative transmembrane domains (see Figure I) as opposed to six for Kv channels or two for KIR channels. Hence these channels are sometimes referred to, rather clumsily, as the 4TM/2PK channel family.
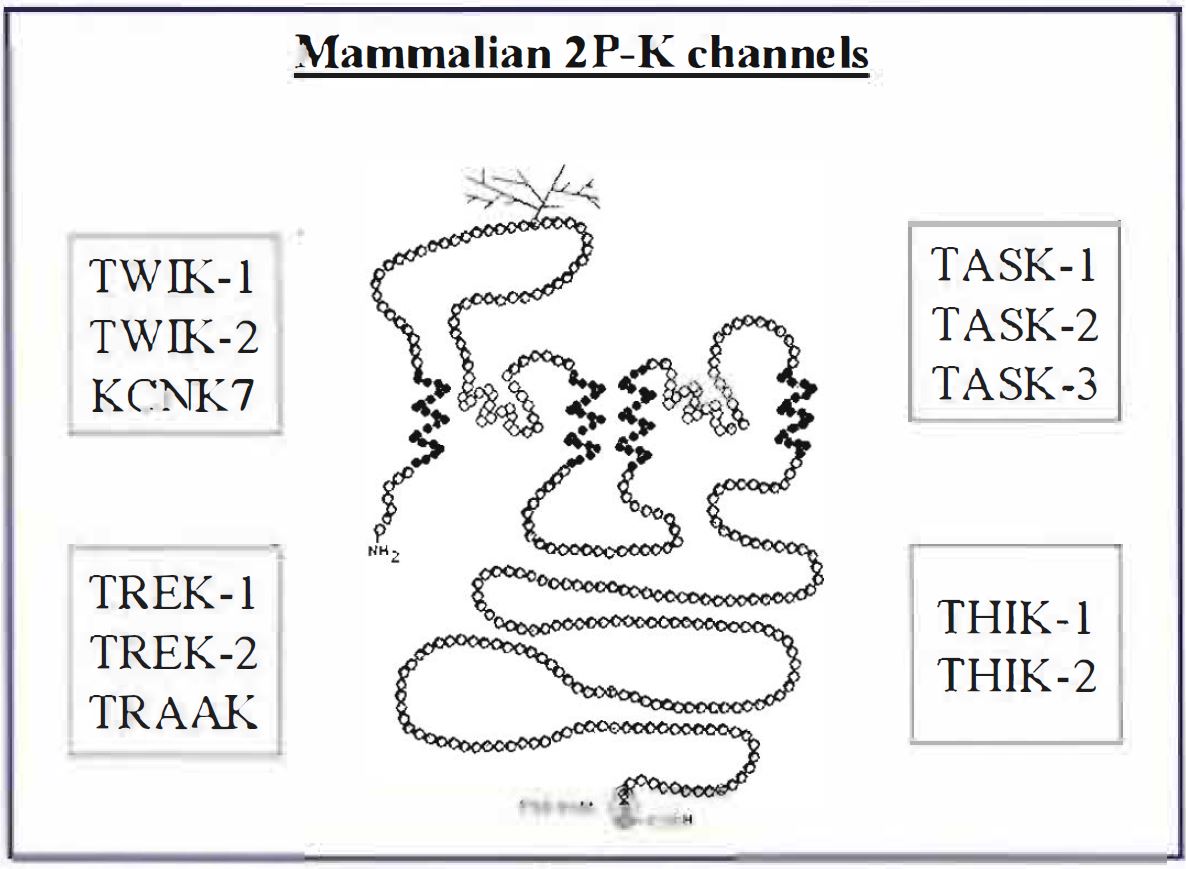
When members of this family of 2P-K channels are functionally expressed, they give rise to K+ selective currents that, generally, are open at all voltages. Most (except, perhaps, TWIK-1 and TWIK-2) show little rectification other than that resulting from the uneven distribution of potassium ions across membranes, that occurs physiologically and which gives rise to an outward rectification often termed “open” or “Goldman-Hodgkin-Katz” rectification (see Goldstein et al., 2001 ). An important diagnostic feature of the currents through 2P-K channels is that they are resistant to block by the classical K channel blocking drugs, tetraethylammonium ions (TEA) and 4-aminopyridine (4-AP). Thus currents through these channels have all the hallmarks of leak or background K channels that are found in most, if not all, eel Is and which, for many electrophysiologists, are nearly (but not quite) removed routinely from their experiments with PIN leak subtraction protocols.
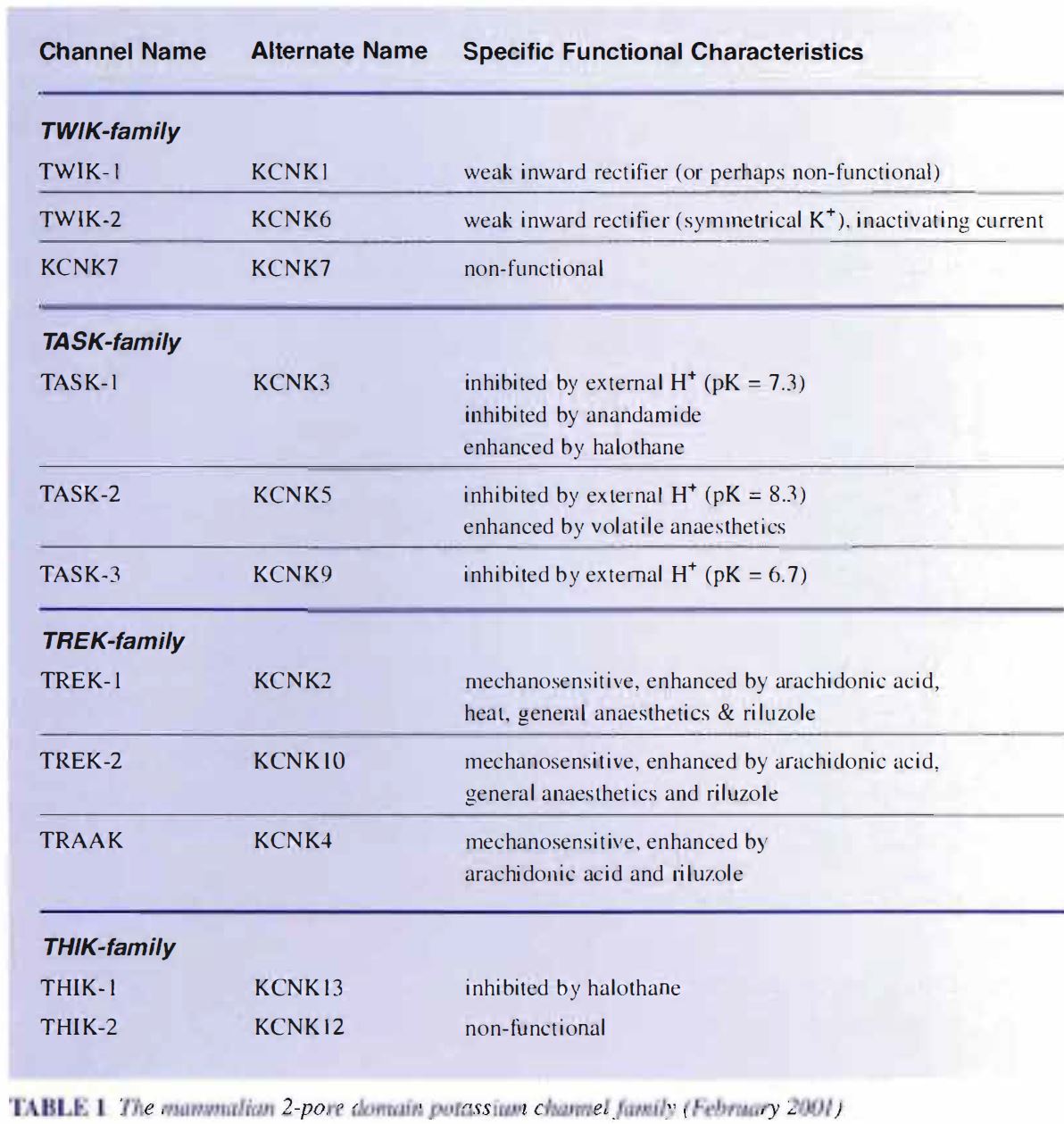
At the time of writing (February 2001) there are known to be l l mammalian genes that encode for channels in this family, but there are certainly more to come. Just how many more remains an open question. They can be divided, largely on the strength of differing functional properties, into four subfamilies. The TWIK family (TWIK- 1, TWIK-2 and KCNK7), the TASK family (TASK-I, TASK-2 and TASK-3; although TASK-2 is structurally very different from TASK-1 and TASK-3), the TREK family (TREK- 1, TREK-2 and TRAAK) and the THIK family (THIK-1 and THIK-2) – see Table 1. Some of these channels do not give functional currents when expressed in artificial systems but the precise number of non-functional channels is not completely clear. Certainly, KCNK7 and THIK-2 are non-functional, however, there is some debate as to whether sole expression of TWIK- l (ironically the first mammalian 2P-K channel to be cloned) gives rise to functional channels. There is also some debate about what nomenclature should be used to describe these channels, with two excellent recent reviews by arguably the two leading protagonists in the field (Lesage & Lazdunski, 2000; Goldstein et al., 200I) expressing different views. The choice is between the TWIK/TASK nomenclature or the KCNK l/KCNK2 nomenclature. For what it is worth, for now, we favour the TWIK/TASK nomenclature. To us, at least, it is easier to remember each channel as an individual using four letter words.
The main functional differences between the different subfamilies of these channels reflect their regulation by different chemical entities (see Table 1). So, for example, the TASK family are highly sensitive to changes in extracellular pH, the TREK family are enhanced by arachidonic acid and mechanical stretch and while the activity of some of the channels in both of these families are enhanced by certain volatile general anaesthetic agents (Patel et al., 1999); the THIK family (at least THIK-1) are inhibited by the general anaesthetic agent halothane (Rajan et al., 200 I). Recently, some of these channels (TASK], TREK-2) (Czirjak et al., 2000; Millar et al., 2000; Talley et al., 2000; Lesage et al., 2000) have been shown to be regulated by G-protein coupled receptors such as muscarinic acetylcholine receptors, angiotensin II receptors, TRH receptors and metabotropic glutamate receptors. It is this plethora of regulatory pathways that make these channels so exciting. Up until now, these currents have tended to be seen as constant, stable (perhaps rather boring) background currents, acting simply to decrease cell input resistance and dampen excitability.
Now, because many regulatory pathways have been shown to act on these 2P-K channels from second to second to alter their activity, these channels can now be seen to have the potential to determine, absolutely, the excitability of a cell and how this varies both with time and with the recent experiences of that cell.
From a physiological perspective, a primary interest lies in determining which cells possess which 2P-K channels, whether they are important contributors to the background currents in these eel Is and if they are, determining how the excitability of that cell will be regulated. Since many of the cloned channels have only been described in the last eighteen months or so, not surprisingly, there is not a huge amount of information as to either which channels are expressed in which cells, or which of them are functionally important for a given cell. To date, best progress has been made for TASK- I. Four groups (including our own) almost simultaneously described four cell types where native background K channels displayed all the hallmarks of expressed TASK-1 channels. These are native currents in cerebellar granule cells (originally termed IKso), hypoglossal motoneurons, arterial chemoreceptor cells and glomerulosa cells of the adrenal cortex (Millar et al., 2000; Talley et al., 2000; Buckler et al., 2000; Czirjak et al., 2000).
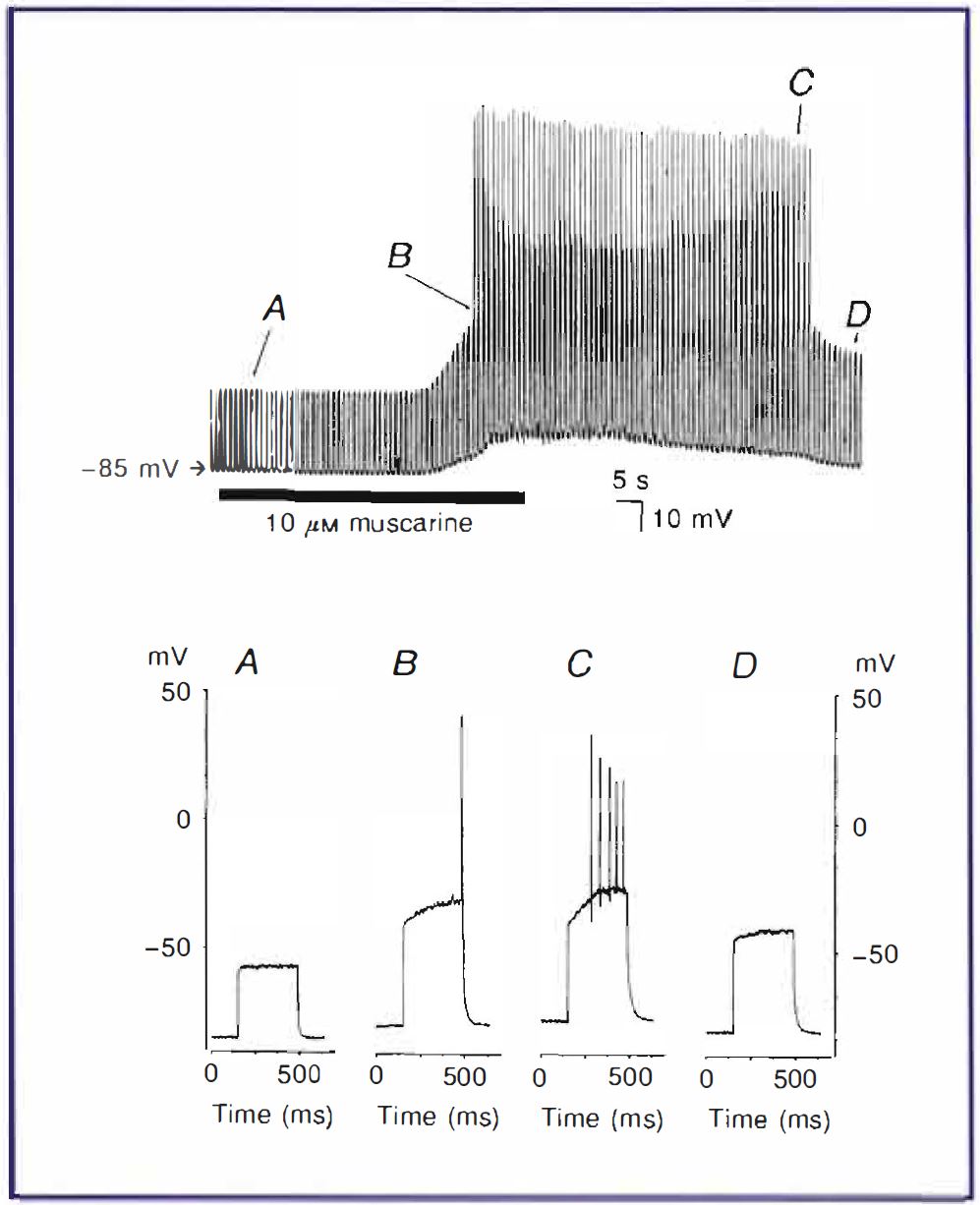
Focusing on our own work, cerebellar granule cells present a particular problem with such a correlation between native and cloned channels since, at the mRNA level at least, they seem to express a number of different 2-PK channels. One of these, the TASK-3 channel, is functionally very similar to TASK-I. Recent studies have backed up the view that TASK-1 channels are crucial to granule cell excitability (Brickley et al., 200 I; Maingret et al., 2001) but a contribution ofTASK-3 channels either on their own or expressed as heterodimers with TASK-I channels cannot be ruled out. The importance of 2P-K channels in regulating granule cell neuronal excitability is illustrated by the experiment shown in Figure 2, where a given amount of current injection can only excite the granule cell to fire action potentials when the endogenous TASK-like current (IKso) is blocked following activation of M3 muscarinic acetylcholine receptors (see also Watkins & Mathie, 1996).
A recent paper has suggested that the background K+ current in cardiomyocytes can be attributed to the 2P-K channel, TREK-1 (Aimond et al., 2000) but there are no defined native correlates for any of the other cloned 2P-K channels so far. In some cells it may prove difficult to define the exact 2P-K channel(s) underlying a native background current. For example, in cerebellar Purkinje cells, we have observed a background K+ current that shares some of the properties of cloned 2P-K channels but it is not possible to determine which 2P-K channel is predominant (Bushnell et al., 2000). This may reflect either a multiplicity of expressed 2P-K channels in these cells or else simply be a result of expressed 2P-K channels not behaving the same way in artificial expression systems as they would do in their native environment.
In short, 2P-K channels represent an important new family of K channels. The activity of these channels and how this varies with time is crucial to the regulation of cell excitabiIity. For us as physiologists, the immediate challenge is to determine which of these channels are present and functionally important in the particular cells that torment us.
Acknowledgements
Our work on 2P-K channels is supported by the MRC, BBSRC and the Wellcome Trust.
References
Aimond, F. et al. (2000). Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+
current !TREK in cardiomyocytes. J. Biol. Chem. 275 39110-391 I 6.
Brickley, S. et al. (2001 ). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409 88-92.
Buckler, K.J. et al. (2000). An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 525 135-142.
Bushell, T. et al. (2000). Pharmacological characterisation of the ILEAK present in mouse cerebellar Purkinje neurons in vitro. J. Physiol. 28 55P.
Czirjak, G. el al. (2000). TASK (TWIK related acid sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mo/. Endocrinol. 14 863-874.
Goldstein, S.A. el al. (200 I). Potassium leak channels and the KCNK family of two-P-domain subunits. Nature Reviews Neuroscience 2 175-184.
Gray, A.T. (2000). New diversity within the mammalian tandem pore domain K+ channel family. Modulalator 13 5-7.
Lesage, F. & Lazdunski, M. (2000). Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. (Renal) 279 F793-F801.
Lesage, F. el al. (2000). Human TREK-2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids and G,, G; and Gq protein-coupled receptors. 1. Biol. Chem. 275 28398-28405.
Maingret, F. et al. (2001). The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-I. EMBO J. 20 47-54
Millar, J.A. el al. (2000). A functional role for the two-pore domain potassium channel TASK-I in cerebellar granule neurons. P.N.A.S. 97 3614-36 I 8.
Patel, A. J. el al. (1999). Inhalation anesthetics activate two-pore domain background K+ channels. Nature Neurosci. 2 422-426.
Rajan, S. el al. (2001). THTK-1 and THTK-2, a novel subfamily of tandem pore domain K channels. J. Biol. Chem. 276 7302-7311.
Talley, E.M. el al. (2000). TASK-I, a two-pore domain potassium channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25 399- 410.
Watkins, C.S. & Mathie, A. ( 1996). A noninactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J. Physiol. 491 401-412.
