
Physiology News Magazine
Managing magnitude and duration of amino acid availability as a strategy to optimize protein gain?
The effects of the pattern of amino acid availability on protein metabolism suggest that this factor might be used to optimize protein gain in various circumstances
Features
Managing magnitude and duration of amino acid availability as a strategy to optimize protein gain?
The effects of the pattern of amino acid availability on protein metabolism suggest that this factor might be used to optimize protein gain in various circumstances
Features
Martial Dangin, Yves Boirie, & Pierre Gachon
Centre de Recherche en Nutrition Humaine Clermont-Ferrand, France
https://doi.org/10.36866/pn.52.31

It has long been recognized that optimization of protein retention in various circumstances requires to consider numerous parameters such as nutritional factors. Main dietary parameters that influence protein metabolism include the amount, the composition and the digestibility of dietary proteins as well as the dose and the composition of non-protein sources (fat, carbohydrates). More recently, human studies have provided new insight into the factors modulating protein metabolism and consequently protein retention. The bio-availability of dietary amino acids over time is one of them. It can be modified either by adapting rate of digestion or changing pattern of feeding.
With respect to digestion rate, the two major bovine milk protein fractions, i.e. whey protein and casein, have quite different behaviour (Boirie et al. 1997). Whey protein that remains in a liquid form in the stomach is emptied into the duodenum and appears rapidly in the blood after intestinal absorption of its constitutive amino acids. By contrast casein, that makes a clot at low pH in the stomach, is emptied much more slowly and the rate of delivery of its amino acids in the blood is slower, lower and more prolonged. The pattern of amino acid availability, as reflected by plasma aminoacidemia, is similar to the one of the appearance of dietary amino acids. With fast digested proteins, such as whey protein, the elevation of amino acid availability is high but of short duration while with a slow one, such as casein, the increase is lower but more persistent. ‘Fast’ protein induces a transient stimulation of protein synthesis, a dramatic increase of amino acid oxidation and has minimal effect on proteolysis. It is quite different with a ‘slow’ protein: protein synthesis is unaffected, amino acid oxidation increases moderately and proteolysis is inhibited for a long period. Finally, protein balance is lower with a fast digested protein, than with a slow one (Fig.). Taken together, these results suggest that protein digestion rate affects protein gain by modulating amino acid availability (Boirie et al. 1997).
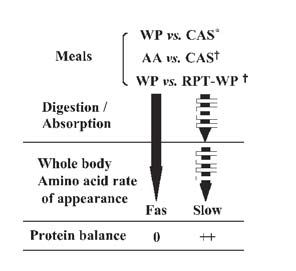
This interpretation is fully confirmed by two set of experiments (Dangin et al. 2001). Indeed, when casein (‘slow’ protein) is compared with a mixture of free amino acids mimicking the composition of casein (rapidly digested) or when a single whey protein meal (‘fast’ protein) is compared with a sequence of small meals made of whey protein and given every 20 minutes (paradigm for a ‘slow’ protein), the results are consistent with those above mentioned. Indeed, in the two pairs of studies, ‘fast’ digested meals stimulate protein synthesis and amino acid oxidation and induce a lower protein gain than “slow” digested ones that inhibit proteolysis durably and increase amino acid oxidation to a lower extent (Fig.). Since the meals differ only by their digestion rate, their amino acid content being identical, protein digestion rate independently modulates protein gain (Dangin et al. 2001).
The key factors responsible for the effects of protein digestion rate on protein anabolism appear to be mainly amino acid availability. Indeed, it is known that amino acid availability, within a physiological range, affects, as a dose dependent manner, protein synthesis and amino acid oxidation (Giordano et al. 1996). For proteolysis, the differential effects of ‘slow and fast’ proteins may be attributed to the duration of the hyperaminoacidemia, because amino acids are known to inhibit proteolysis, which is more prolonged with ‘slow’ than with ‘fast’ proteins. However, all these results have been obtained after ingestion of protein meals without any non-protein energy, factor known to possibly affect protein metabolism.
Modulation of amino acid availability can be obtained by ingesting ‘slow or fast’ protein, but it can also be modified by changing the pattern of feeding over the day. For example, it is known that total parenteral nutrition provided as bolus or as constant infusion affects plasma aminoacidemia and nitrogen balance according to the physiological state of the subjects. It has also been reported, in young healthy women (Arnal et al. 2000), a trend of higher nitrogen balance with a ‘spread’ feeding pattern, i.e. daily protein intake evenly distributed over four meals, than with a ‘pulse’ one (80% of the daily protein intake consumed at noon). Since one might consider that the spread feeding pattern induces a lower but more prolonged rise in plasma aminoacidemia than the pulse one, these results are in agreement with those obtained after ‘slow and fast’ protein ingestion. In contrast, in elderly women, the results are quite different: the pulse diet induces a better nitrogen balance (Arnal et al. 1999). This effect appears to be to age-related, since there is a greater efficiency of a ‘fast’ protein than a ‘slow’ one in improving protein gain in elderly men, in contrast with the younger group, when consuming mixed meals (Dangin et al. 2003). In addition, it is described that stimulation of protein synthesis of old muscle is impaired at low increment in amino acid availability, but that response can be normalized by high levels of amino acids and/or leucine.
Collectively, these studies support the general idea that the magnitude and the duration of change in amino acid availability regulate protein gain after meal ingestion. That factor can be controlled by choosing protein with specific digestion rates, adapting the infusion rate of nutrients or by changing protein feeding pattern. It may thus represent an adjunctive dietary strategy to optimize protein gain in various pathological circumstances. However, since these effects can be modulated by extrinsic factors such as age or metabolic stress or by intrinsic factors such as non protein nutrients in the meals, their implications for human nutrition warrant further examinations.
References
Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrère B & Mirand PP (1999). Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr 69, 1202-1208.
Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrère B & Mirand PP (2000). Protein feeding pattern does not affect protein retention in young women. J Nutr 130, 1700-1704.
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL & Beaufrère, B. (1997). Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94, 1493014935.
Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballèvre O & Beaufrère B (2001). The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280, E340-E348
Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O & Beaufrère B (2003). Protein digestion rate affects differently protein gain during aging. J. Physiol(Lond) 549, 635-644.
Giordano M, Castellino P, DeFronzo RA (1996). Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes 45, 393-399.
