
Physiology News Magazine
Melatonin, sleep and the biological clock
Jo Arendt cuts through the hype and looks at the genuine benefits of a ‘hormone of darkness’
Features
Melatonin, sleep and the biological clock
Jo Arendt cuts through the hype and looks at the genuine benefits of a ‘hormone of darkness’
Features
Josephine Arendt
Centre for Chronobiology, School of Biomedical and Molecular Sciences, University of Surrey, Guildford, Surrey, UK
https://doi.org/10.36866/pn.61.22
Humans are a diurnal species, active during the day, sleeping at night. In optimal conditions alternating light and darkness dictate the timing of our endogenous clock (the central circadian pacemaker, the suprachiasmatic nuclei or SCN) which in turn synchronises our physiology such that systems work in harmony with the environment. We sleep better when the internal clock is in the right phase. But in developed countries we live, most of us, in an artificial environment. Alarm clocks wake us up, not the sunrise. Thanks to Edison we do not go to bed at sunset. Our homes, cities and roads are illuminated and many of us (around 20% of the UK work force) work at night and attempt to sleep during the day usually at odds with internal physiology. We subject ourselves to abrupt large shifts in time cues when flying long haul. Our biology has not caught up with our technology: the clock does not adapt rapidly to night work (indeed if at all) or time zone change.
Even if people are day active the clock can drift away from its normal phase (usually by delay) with insufficient time cues. The most important of these is sufficiently bright light of suitable spectral composition (short wavelengths are most effective). This is particularly evident in the Polar winter (no sunlight at all), but may be prevalent in urban indoor workers, especially those with a strong diurnal preference for evening (owls). Delayed Sleep Phase Syndrome (DSPS) is a manifestation of extreme delay. As a result of suboptimal timing of the internal clock, at the very least we frequently get insufficient, poor quality sleep. Shift workers in particular have short sleep, taken at an inappropriate phase of the clock. At worst, living counter to the internal clock may lead to increased risk of major disease, including heart disease and cancer, and even the current obesity epidemic may be related in part to insufficient good quality sleep.
So where does the pineal hormone melatonin come in to this? Reading some of the scientific literature one might be forgiven for concluding that melatonin was the ‘hormone of sleep’. It is, in fact, a ‘hormone of darkness’, effectively an internal time cue. It is normally made at night in all species, whether nocturnal or diurnal. Its secretion pattern reflects the length of the night and serves to define ‘biological night’. Thus in humans, (but not in rats) its peak values are normally associated with sleep and the nadir of core body temperature, alertness and performance and other phenomena characteristic of night time. The rhythm is driven by the SCN and, like all circadian rhythms, its timing is dictated largely by the light dark cycle. The melatonin rhythm (or that of its major metabolite 6-sulphatoxymelatonin) is the best peripheral index of biological clock timing and is used extensively to determine human circadian timing in health and disease.
Suppression of melatonin by light at night has provided a tool for investigation of circadian photoreception, a hypothesis for the health risks of shift work, and the rationale for the first light treatments of seasonal depression (SAD). The major physiological role of melatonin is to convey information about light and darkness: it is the only solidly established humoral method of signalling time of day and time of year to other physiological systems.
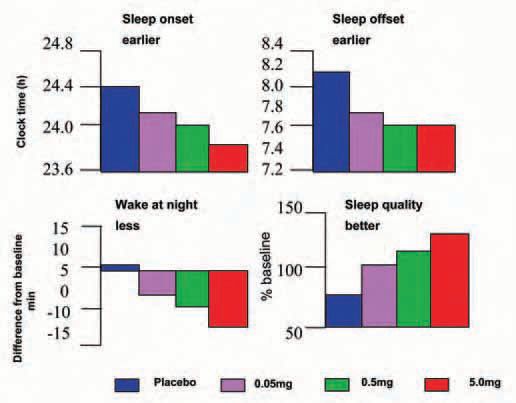
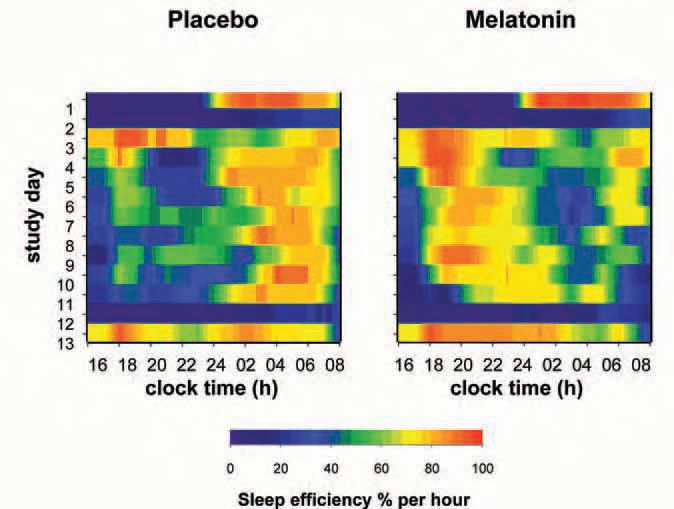
Pinealectomy abolishes the melatonin rhythm- and the ability to perceive daylength changes: a major problem for daylength dependent seasonal species. Humans retain some seasonality (even in fertility) and some of the actions of melatonin in humans can be interpreted on this basis. In physiological or near to physiological doses, it has two main effects in humans. Taken during ‘biological daytime’ it induces sleepiness and lowers body temperature in the hours following ingestion, if subjects are recumbent or semirecumbent in dim light. Taken during biological afternoon-evening it facilitates sleep and advances the timing of all circadian rhythms measured to date, probably by a direct action on receptors in the SCN (Fig. 1). Taken in the biological evening in a person whose clock has drifted into a phase which is too late for optimal sleep at the conventional time, the phase advance may optimise the timing of the clock relative to the desired sleep time. It is also reported to delay the circadian system if taken during early ‘biological morning’ but this is more controversial. Its effects on sleep, within an extended ‘sleep opportunity’ (Fig. 2), suggest that it does not grossly alter sleep structure or total sleep time but redistributes sleep in a manner reminiscent of the redistribution of sleep in long and short nights in animals.
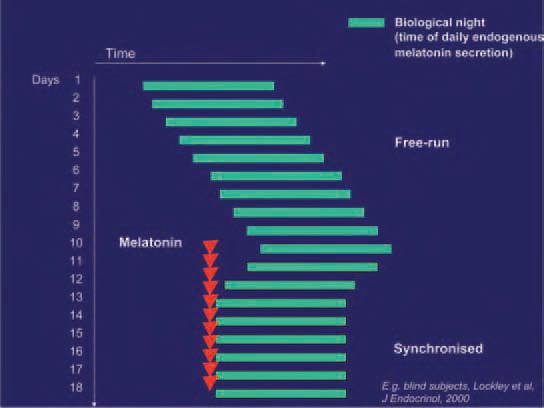
In the absence of strong time cues the circadian system desynchronises completely from 24h clock time and assumes the genetically determined endogenous period (usually longer than 24h) of the individual concerned (‘freerunning’). This condition is particularly common in blind people with no light perception at all. In most cases melatonin is able to synchronise such free running circadian rhythms to 24 h with suitable timing and dose (Fig. 3).
These ‘chronobiotic’ properties, together with its acute effects on sleep, mean that melatonin is used successfully to correct circadian rhythm abnormalities such as free-running sleep disorder of the blind, DSPS, and, less consistently, the sleep problems of jet lag and night shift work. The extensive use of melatonin as a sleep aid in, for example, the USA rests probably on the correction by evening melatonin of a tendency to delayed phase. With suitable dose and timing both the acute and phase shifting effects of melatonin can be maximised. These properties have inspired new pharmacological approaches to the treatment of health problems and it is possible that optimisation of circadian timing will have many health benefits yet to be determined.
Since melatonin has powerful physiological effects in photoperiodic species we must be certain of its uses and limitations. There is little information on long term safety even though we have known for more than 40 years that melatonin induced sleepiness (as reported by Aaron Lerner who discovered melatonin) and might be clinically useful. However, there is very little evidence in the short term for toxicity or undesirable effects in humans. Indeed there is accumulating evidence for anti-cancer properties and other potential benefits. The extraordinary ‘hype’ of the miraculous powers of melatonin in the recent past did a disservice to acceptance of its proven therapeutic uses.
References and further reading
Arendt J (2000). Melatonin, circadian rhythms, and sleep. Editorial. N Engl J Med 343, 1114-1116.
Arendt J & Skene DJ (2004). Melatonin as a chronobiotic. Sleep Medicine Reviews 9, 25-39.
Arendt J (2005). Melatonin: characteristics, concerns and prospects. J Biol Rhythms 20, 291-303.
Deacon S & Arendt J (1995). Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dosedependent manner in humans. Brain Res 688, 77-85.
Lockley SW, Skene DJ, James K, Thapan K, Wright J & Arendt J (2000). Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 4, R1-6 .
Rajaratnam SM & Arendt J (2001). Health in a 24-h society. Lancet 358, 999-1005.
Rajaratnam S M, Dijk D J, Middleton B, Stone B & Arendt J (2003). Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-hour production of reproductive hormones. J Clin Endocrinol Metab 88, 4303-4309.
Rajaratnam SMW, Middleton B, Stone BM, Arendt J & Dijk D-J (2004). Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities. J Physiol 561, 339-351.
Revell VL, Arendt J, Terman M & Skene DJ (2005). Shortwavelength sensitivity of the human circadian system to phase advancing light. J Biol Rhythms 20, 270-272.
Thapan K, Arendt J & Skene DJ (2001). An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535, 261-267.
Warman VL, Dijk D-J, Warman GR, Arendt J & Skene DJ (2003). Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett 342, 37-40.
