
Physiology News Magazine
Mice deficient in endothelin-3 gene lack pain perception from the terminal gastrointestinal tract
In many different types of mammal, endothelin peptides and their endogenous receptors have been shown to play a major role in nociception in a variety of visceral and somatic organs. Here, we report that mice with a genetic mutation in the endothelin-3 gene have a major sensory deficit in a specific class of low-threshold wide dynamic range spinal afferent nerve that innervates the colorectum. Functionally, these mice display a loss of mechano-nociception selectively from the colorectum, whilst pain perception from other internal and somatic organs remains unaffected.
Features
Mice deficient in endothelin-3 gene lack pain perception from the terminal gastrointestinal tract
In many different types of mammal, endothelin peptides and their endogenous receptors have been shown to play a major role in nociception in a variety of visceral and somatic organs. Here, we report that mice with a genetic mutation in the endothelin-3 gene have a major sensory deficit in a specific class of low-threshold wide dynamic range spinal afferent nerve that innervates the colorectum. Functionally, these mice display a loss of mechano-nociception selectively from the colorectum, whilst pain perception from other internal and somatic organs remains unaffected.
Features
Nick J. Spencer, Simon J. Brookes & Vladimir P. Zagorodnyuk
Discipline of Human Physiology, Flinders Medical Science and Technology Cluster, Flinders University, South Australia, 5001, Australia
https://doi.org/10.36866/pn.85.26



Intense distension or contraction (spasm) of different regions of the gastrointestinal (GI) tract is well known to lead to the conscious sensation of pain in both laboratory animals and human beings. However, identifying exactly which specific classes of sensory fibres detect painful stimuli in vivo throughout the different regions of the GI tract has been difficult to elucidate. Pain arising from all internal (visceral) organs is detected and transmitted to the spinal cord via spinal afferent neurons, whose cell bodies lie in the dorsal root ganglia. Consequently, there has been substantial clinical interest in identifying exactly which classes of spinal afferent could be targeted to develop therapies that might selectively target and silence ‘pain-sensing’ neurons. Unfortunately, this has been a difficult question to address because it is clear that the spinal afferent innervation of the lower GI tract contains at least four to five different classes of sensory fibre, each of which responds to different types and intensities of stimulation (Brierley et al. 2004).
Substantial evidence has been presented that endogenous endothelins and their receptors participate in a wide range of nociceptive behaviours (Baamonde et al. 2004; Khodorova et al. 2009; Stösser et al. 2010). It is also well known that genetic mutations that lead to a loss of endothelin peptides, or their cognate receptors, lead to developmental defects in the gut wall such as loss of intrinsic neurons in the terminal gastrointestinal tract – a condition known as congenital aganglionosis, or Hirschsprung’s disease in humans.

Our recent work has focused on the spinal afferent innervation of the colorectum in a mutant strain of mouse that is deficient in a gene known as endothelin-3 (ET-3). This gene encodes production of the endothelin peptide, whose function in sensory neurophysiology in the gastrointestinal tract has been difficult to establish. However, we recently made a major advance in our laboratory in identifying that mice lacking the ET-3 gene fail to detect pain following noxious colorectal distension (see Fig. 1 and Zagorodnyuk et al. 2011). Only at the highest distension pressures tested was any pain response elicited in ET-3-deficient mice (Fig.1). In contrast, robust visceromotor responses (VMRs) could be evoked in wild type mice with stimuli over 20 mmHg. An interesting observation we found in our study was that this sensory deficit was specific for the colorectum (Zagorodnyuk et al. 2011). In other words, loss of pain perception from the colorectum did not reflect a generalized deficit in central sensory or motor pathways, since responses to both bladder distension (a visceral stimulus) and tail and hind limb compression (somatic stimuli) were normal in ET-3-deficient mice (see Fig. 2 and Zagorodnyuk et al. 2011). Thus, the deficit lay specifically in the sensory pathways to the distal bowel of ET-3-deficient mice. We then tested whether the neural pathway that normally detects and encrypts pain perception was present and intact in colorectum of ls/ls mice. To test this, we applied fine electrical stimulating wires to the surface of the aganglionic colorectum (in vivo) and found we could still evoke visceromotor responses in ls/ls mice, albeit these responses were significantly smaller than those in wild type mice. This showed that the loss of pain perception was not due to the loss of a pain pathway or spinal circuitry required for pain reflexes in vivo.
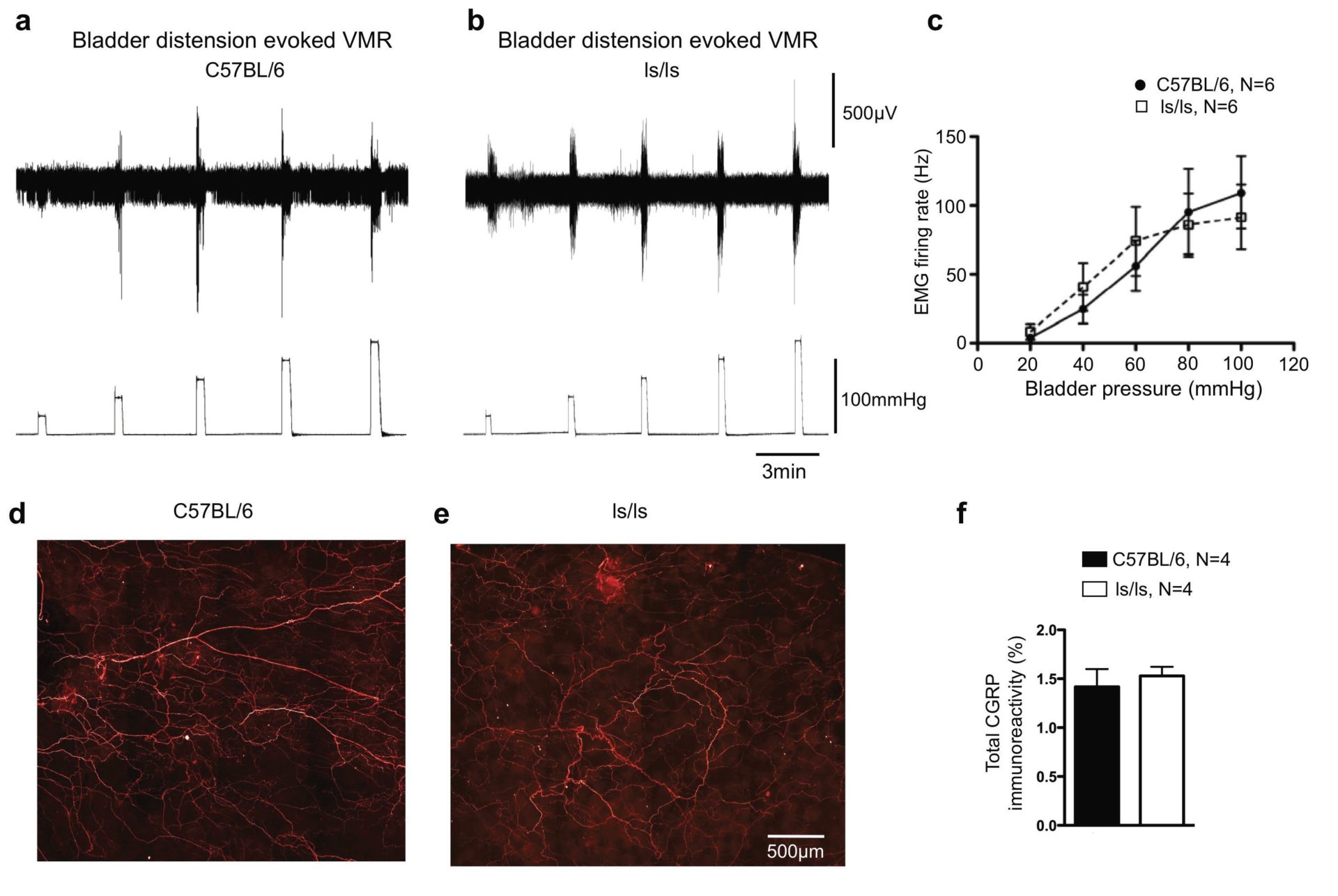
In earlier studies we had shown that low-threshold, stretch-sensitive rectal afferents innervated the smooth muscle of the aganglionic colorectum in mice with mutations in either the gene encoding ET-3 or the endothelin-B receptor (Ednrb) (Spencer et al. 2008a,b). We were able to identify which specific classes of afferent might be impaired. We identified that loss of pain perception in ET-3-deficient mice was due to a specific reduction in the density and mechanosensitivity of a class of low-threshold, wide dynamic range stretch-sensitive rectal afferents (Fig. 2). This class of sensory fibre was known as either a muscular or muscular/mucosal afferent, which responded to stretch and/or mucosal stimulation.
Four classes of pelvic/sacral afferents have been identified in the mouse colorectum. These are known as muscular, muscular-mucosal, serosal and mucosal afferents (Brierley et al. 2004). We found that the muscular and muscular-mucosal afferents responded to distension with low thresholds and wide dynamic ranges which did not saturate across a wide range of stretch amplitudes. Serosal afferents had significantly higher thresholds. This is relevant because visceromotor responses evoked by noxious colorectal distension were consistently activated at distension pressures in the range of 20–40 mmHg. However, serosal afferents had thresholds of 10–20 g circumferential load, which corresponded (using the Young–Laplace law) to intraluminal pressures of 50–100 mmHg. In contrast, low-threshold wide dynamic range fibres had thresholds below 3 g (~15 mmHg). This suggests that low-threshold, wide dynamic range fibres are likely to carry most of the afferent signal involved in visceromotor responses. Thus, high-threshold (serosal) afferents (Song et al. 2009) are likely to play a much smaller role in distension-evoked visceromotor responses from the colorectum. Even at maximum stretch (20–40 g which corresponds to 100–200 mmHg using the Young–Laplace law) serosal units contribute only 5% of total stretch-induced firing from the colorectum. Taken together, these findings suggest a major role for rectal low-threshold wide dynamic range mechanoreceptors in the generation of VMRs to colorectal distension.
Exactly how disruption of endothelin-3 signalling causes changes in spinal afferent density and mechanosensitivity remains unclear. Recent studies have shown that family members of glial cell line-derived neurotrophic factor (GDNF), which are known to be essential for migration of the enteric neural crest during development of the enteric nervous system (Heanue & Pachnis, 2007), also play a major role in visceral hypersensitivity and sensitization of muscular and muscular-mucosal colorectal afferents (Tanaka et al. 2010). It is intriguing to speculate that endothelin-3 may play a trophic role in the development and/or survival of low-threshold stretch-sensitive afferents involved in rectal nociception.
References
Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A & Menendez L (2004). Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn-Schmiedebergs Arch Pharmacol 369, 245–251.
Brierley SM, Jones RC 3rd, Gebhart GF & Blackshaw LA (2004). Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178.
Heanue TA & Pachnis V (2007). Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cells studies. Nat Rev Neurosci 8, 466–479.
Khodorova A, Montmayeur JP & Strichartz G (2009). Endothelin receptors and pain. J Pain 10, 4–28.
Song X, Chen BN, Zagorodnyuk VP, Lynn PA, Blackshaw LA, Grundy D, Brunsden AM, Costa M & Brookes SJ (2009). Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137, 274–284.
Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT & McDonnell O (2008a). Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 153, 518–534.
Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ & McDonnell O (2008b). Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol Gastrointest Liver Physiol 294, G855–G867.
Stösser S, Agarwal N, Tappe-Theodor A, Yanagisawa M & Kuner R (2010). Dissecting the functional significance of endothelin A receptors in peripheral nociceptors in vivo via conditional gene deletion. Pain 148, 206–214.
Tanaka T, Shinoda M, Feng B, Albers KM & Gebhart GF (2010). Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor (GDNF) family receptor alpha-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol 300, G418–G424.
Zagorodnyuk VP, Kyloh M, Nicholas S, Peiris H, Brookes SJ, Chen BN & Spencer NJ (2011). Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung’s disease. J Physiol 589, 1691–1707. http://jp.physoc.org/content/589/7/1691.long
Acknowledgements
We wish to acknowledge the financial support provided by the National Health and Medical Research Council (NH&MRC) of Australia to grant nos. 535033 and 535034.
