
Physiology News Magazine
Mitochondrial proton conductance in cold adapted king penguins
Keeping warm is a serious business. And there is more to it than fast swimming and a warm coat
Features
Mitochondrial proton conductance in cold adapted king penguins
Keeping warm is a serious business. And there is more to it than fast swimming and a warm coat
Features
Claude Duchamp (1), Darren A Talbot (2), Benjamin Rey (1), Nicolas Hanuise (1), Jean Louis Rouanet (1), Brigitte Sibille (1), & Martin D Brand (2)
1: CNRS-UMR 5123 Université Lyon 1, Laboratoire de Physiologie Intégrative, Cellulaire et Moléculaire, Villeurbanne, France
2: Medical Research Council Dunn Human Nutrition Unit, Cambridge, UK
https://doi.org/10.36866/pn.57.17



Immersion in cold water is one of the most stressful situations encountered by endotherms because of the large thermal gradient and the high thermal conductivity of water. Warm-blooded animals adapted to sea life, such as penguins, must have developed powerful physiological adjustments to withstand this massive energetic challenge by reducing heat loss and by generating heat for regulatory thermogenesis. Heat production through the vigorous physical activity of swimming would not be sufficient as it also raises heat loss severalfold. There is, therefore, more to keeping warm in cold sea water than just physical activity and thermal insulation with fat and dense, waterproof feathers.
The adaptive thermogenic mechanisms developed by marine birds are much less understood than those in mammals, which possess specialized thermogenic brown adipose tissue. In brown fat mitochondria, heat is generated by regulated uncoupling of substrate oxidation and ATP synthesis, catalyzed by a specific mitochondrial protein called uncoupling protein 1 (UCP1). UCP1 increases the proton conductance of the inner membrane and is activated by fatty acids and inhibited by purine nucleoside di- and triphosphates (Cannon & Nedergaard, 2004). Birds lack brown adipose tissue, and instead have uncharacterized adaptive thermogenic mechanisms in skeletal muscle (Duchamp & Barré, 1993). Recently, a homologue of mammalian UCPs has been characterized in avian skeletal muscle, but its biochemical roles remain hypothetical (Raimbault et al. 2001; Talbot et al. 2003).
Are functional UCPs expressed in skeletal muscle from king penguin juveniles? And do they contribute to the adaptive thermogenic mechanisms developed during cold sea adaptation (Barré & Rousset, 1986)? Young king penguins spend their first year on land, protected by a thick insulating down and fed periodically by their parents fishing at sea. Only after moulting at age 12-13 months do they face the thermogenic challenge of passage from shore to marine life in cold subantarctic seawater at 4-6°C. Interestingly, this transition is progressive and requires several acclimation journeys into the sea. This suggests that it involves some specific adaptation that takes time to fully develop.
Talbot et al. (2004) compared three groups of young moulted penguins: juveniles that had never been to sea, juveniles that were naturally adapted to marine life and juveniles that were experimentally exposed to 10 successive 5 hr cold-water immersions at 8°C every second day to reproduce sea acclimatization. Skeletal muscle mitochondria isolated from pectoralis muscle biopsies exhibited two different adaptive mechanisms that increased mitochondrial inner membrane proton conductance and thus are potentially thermogenic: the first involves the avian UCP and the second involves the adenine nucleotide translocase (Fig. 1).
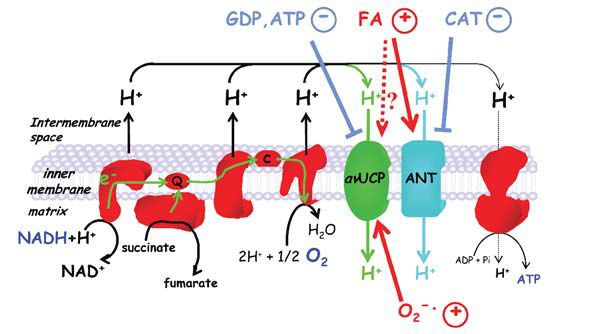
The presence of functional UCP was assessed by the demonstration of superoxide-stimulated, GDP-inhibitable proton conductance across the mitochondrial inner membrane, a common feature of all known UCPs (Echtay et al. 2002; Talbot et al. 2003). Muscle mitochondria from never-immersed juveniles did not have functional UCP, while those from immersed penguins did. Interestingly, experimental immersion in cold water was sufficient to trigger muscle biochemical adaptations. In parallel, the mRNA for penguin UCP was markedly increased in skeletal muscle from both experimentally and naturally immersed juvenile penguins.
A second mechanism catalyzing proton translocation through mitochondrial membranes involves the adenine nucleotide translocase (ANT). In the presence of fatty acids, mitochondria isolated from artificially or naturally immersed juveniles showed a greater carboxyatractylate-sensitive ANT-catalyzed proton conductance than those from never-immersed penguins. This was due to an increase in ANT content as indicated by carboxyatractylate binding and western blots.
Further studies are needed to investigate whether the proton conductances of penguin UCP and ANT are switched on after cold-water immersion as part of thermogenic acclimation and/or to protect against oxidative damage by reducing the production of endogenous reactive oxygen species (ROS) likely to be generated during anoxia/reoxygenation episodes occurring with diving. However, these data represent a breakthrough, as they show for the first time the existence of UCP- and ANT-catalyzed modulation of the proton conductance of the mitochondrial inner membrane in naturally cold-adapted young birds. Further, the fact that such uncoupling of oxidative phosphorylation in skeletal muscle mitochondria can be induced by natural or experimental repeated immersions in cold-water underlines its adaptive value for facing a major cold challenge at a crucial time in penguin life.
Acknowledgements
This work was supported by grants from the Institut Polaire Français Paul Emile Victor (programme 131) and the Medical Research Council.
References
Barré H & Rousset B (1986). Thermal and metabolic adaptation to first cold-water immersion in juvenile penguins. Am J Physiol 251, R456-R462.
Cannon B & Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277-359.
Duchamp C & Barré H (1993). Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol 265, R1076-R1083.
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC & Brand MD (2002). Superoxide activates mitochondrial uncoupling proteins. Nature 415, 96-99.
Raimbault S, Dridi S, Denjean F, Lachuer J, Couplan E, Bouillaud F, Bordas A, Duchamp C, Taouis M & Ricquier D (2001) An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem J 353, 441-444.
Talbot DA, Hanuise N, Rey B, Rouanet JL, Duchamp C & Brand MD (2003) Superoxide activates a GDP-sensitive proton conductance in skeletal muscle mitochondria from king penguin (Aptenodytes patagonicus). Biochem Biophys Res Commun 312, 983-988.
Talbot DA, Duchamp C, Rey B, Hanuise N, Rouanet JL, Sibille B & Brand MD (2004). Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of king penguins. J Physiol 558, 123-135.
