
Physiology News Magazine
Mitochondrial responses to hypoxia and physiological stimulation of harbouring cells measured optically in situ
Understanding the involvement of mitochondria in cellular regulatory processes in living cells and tissues is the hallmark of the current interest in mitochondria. One way to monitor these organelles in live tissue is the newly developed, high-precision, spectral absorption-based method for measuring respiratory pigments’ redox state changes. With it we monitored responses of mitochondria, inside the eyes of live insects, to large perturbations like hypoxia, as well as to more physiological ones such as light stimulation.
Features
Mitochondrial responses to hypoxia and physiological stimulation of harbouring cells measured optically in situ
Understanding the involvement of mitochondria in cellular regulatory processes in living cells and tissues is the hallmark of the current interest in mitochondria. One way to monitor these organelles in live tissue is the newly developed, high-precision, spectral absorption-based method for measuring respiratory pigments’ redox state changes. With it we monitored responses of mitochondria, inside the eyes of live insects, to large perturbations like hypoxia, as well as to more physiological ones such as light stimulation.
Features
Gregor Zupančič and Andrej Meglič
University of Ljubljana, Biotechnical Faculty, Department of Biology, Večna pot 111, 1000 Ljubljana, Slovenia
https://doi.org/10.36866/pn.84.18


One of the limitations to physiological research has always been the availability of tools that would allow monitoring of processes hidden inside other structures – processes in organs within the body, in tissues within organs, in cells within tissues and in cellular organelles within cells. A traditional remedy is to dissect the structure, take out the part of interest and study it in an artificial environment, which is as close as possible to the natural one. This approach has many advantages, especially regarding the vast array of experimental methods that can be employed once the obscuring structure has been removed. It is hardly necessary to state that most of our physiological knowledge has been gained this way. The other approach – to observe the whole body and try to extract meaningful information, from often very distorted signals passing through the many structural layers – has been much less popular, due mainly to its inherent imprecision and artefact-proneness. The middle ground has been covered by approaches using various marker probes – from fluorescent molecular to x-ray contrast ones. There are very few methods available that allow reliable insight down to the molecular level within living organisms without the use of additional artificial markers.
All of the above is certainly true for the study of mitochondria. Yet, as we try to understand their involvement in many cellular regulatory processes, it is becoming increasingly apparent that any methods for monitoring mitochondria, without the need for isolation or introduction of potentially function-interfering markers, would be very useful. We have developed such a method, which allows monitoring of mitochondria by simultaneously measuring the relative redox state changes of all the respiratory pigments present in the respiratory chain (Zupančič, 2003). It is based on the long-known fact that haems, which are part of respiratory complexes, undergo substantial changes of their absorption spectra with reduction and oxidation (Chance & Williams, 1955). There are, however, a few problems associated with this phenomenon.
First, their absorption spectra overlap heavily. Single wavelength measurements of absorption changes are thus doomed to be inaccurate because all the haems change their redox state/absorption simultaneously when mitochondria are activated or experience any other metabolic challenge. The original solution to this problem was to use wavelength pairs whereby one wavelength served as a semi-isosbestic reference while the other reported on the redox state of a particular haem (Chance & Williams, 1955). Although pioneered in the 1950s, this approach is still being used today. Nevertheless, it remains difficult to simultaneously monitor the whole respiratory chain in this way. In addition, this still does not resolve the problem completely since there are no true isosbestic points in the mitochondrial absorption spectra.
Our solution was to use fast spectral imaging of whole tissue absorption spectra by a 2048 element linear CCD array and then numerically post-process the acquired time-coded spectral matrix. We used a combination of digital filtering followed by spectral down-sampling, followed by singular value decomposition (or principal component analysis) and fitting of linear combinations of reference haem spectra to the most significant spectral components. The time courses of respiratory pigments’ reduction/oxidation were finally obtained by multiplying the matrix of the most significant time course vectors with the matrix of fit parameters (for a detailed explanation see Zupančič, 2003). This approach is only possible because, although cytochrome absorption spectra differ between species, the haem difference absorption spectra are remarkably constant from bacteria to man. Because of this, we could use published haem difference absorption spectra from different species as our references. The side benefit of all this numerical processing was a drastic increase in the signal-to-noise ratio in longer records, where it was improved several hundred times, with respect to the original record (Fig. 1).
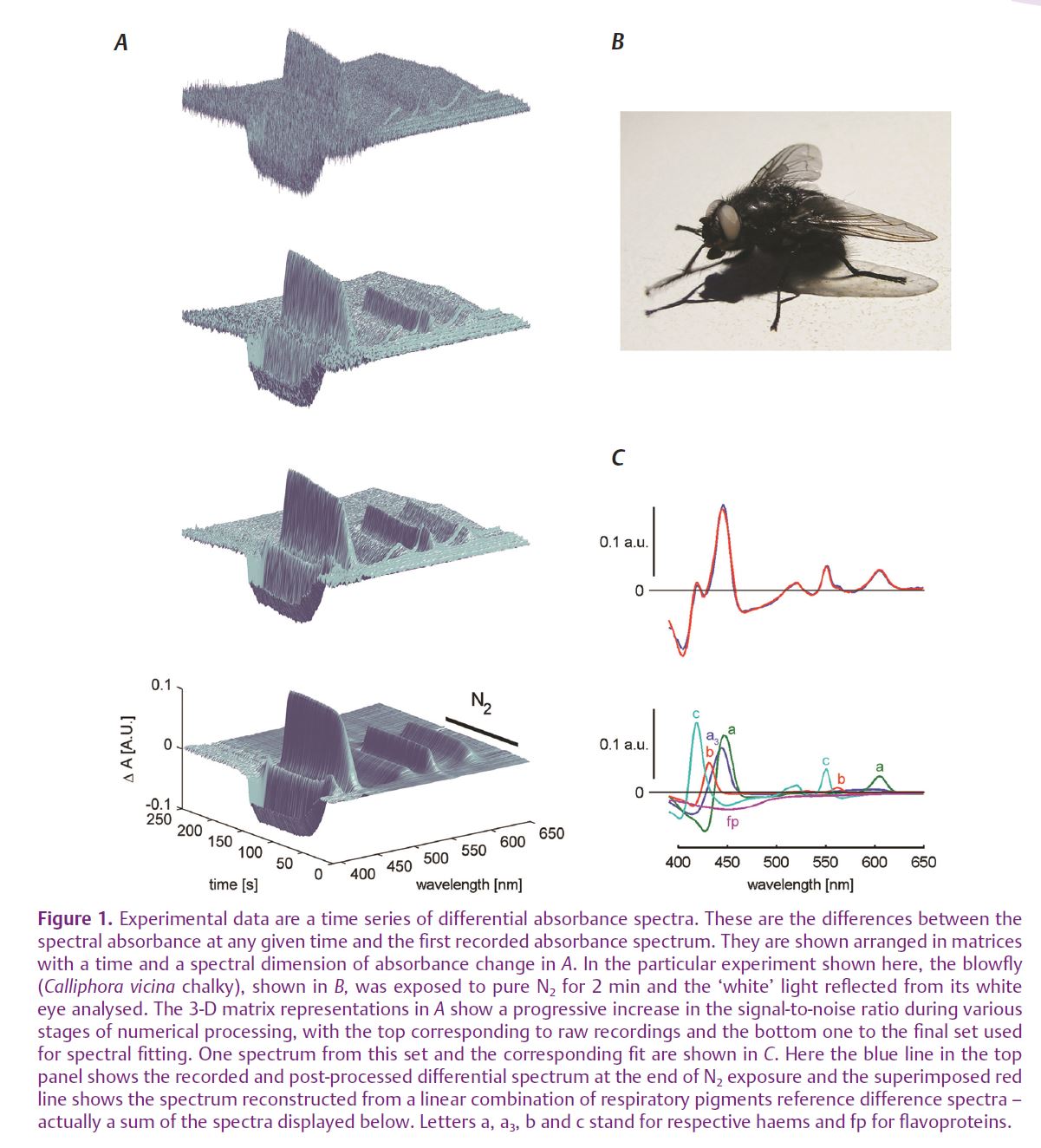
Using this method we were able to examine in great detail what is happening to mitochondria inside a living, although immobilised, animal during various stages of hypoxia, as well as during metabolic challenges associated with normal cellular function (Meglič & Zupančič, 2011). We used mutant white-eyed blowflies that have no screening pigment in their otherwise normally functioning compound eyes. Although this feature prevents the animals seeing a sharp picture of their surroundings, it is very useful for optical measurements of tissue absorption changes. Light that enters the eye is scattered many times by the many interfaces between the air in the tracheal system and the tissue, and a substantial portion of it leaves the eye, making it appear white. This light, however, bears also the signature of all light-absorbing molecules present in the eye – rhodopsin/meta rhodopsin, cytochromes, flavines, NADH/ NAD. The respiratory pigments are especially abundant, since the photoreceptor cells are full of mitochondria, which support the ion pumps in maintaining the ionic imbalance in the presence of strong light-induced ionic currents.
The insect phototransduction is in contrast to vertebrates coupled to TRP and TRPL channel-mediated depolarization upon illumination, via G q-protein and PLCb (Hardie, 2007). The one in flies is usually exemplified as the fastest known G-protein-coupled cascade due to its high degree of spatial organization. Our experiments were made easier, on one hand, by the fact that the tracheal system brings air virtually to each cell. Since the O2 diffusion in the air is approx. 10,000 times faster than in water, this allows fast changes in O2 content deep within the eye tissue. On the other hand, the very high speed of the transduction cascade produces close to step changes of many cellular physiological parameters – membrane potential, [Ca2+]i, etc. (Gerster et al. 1997; Oberwinkler & Stavenga, 2000).
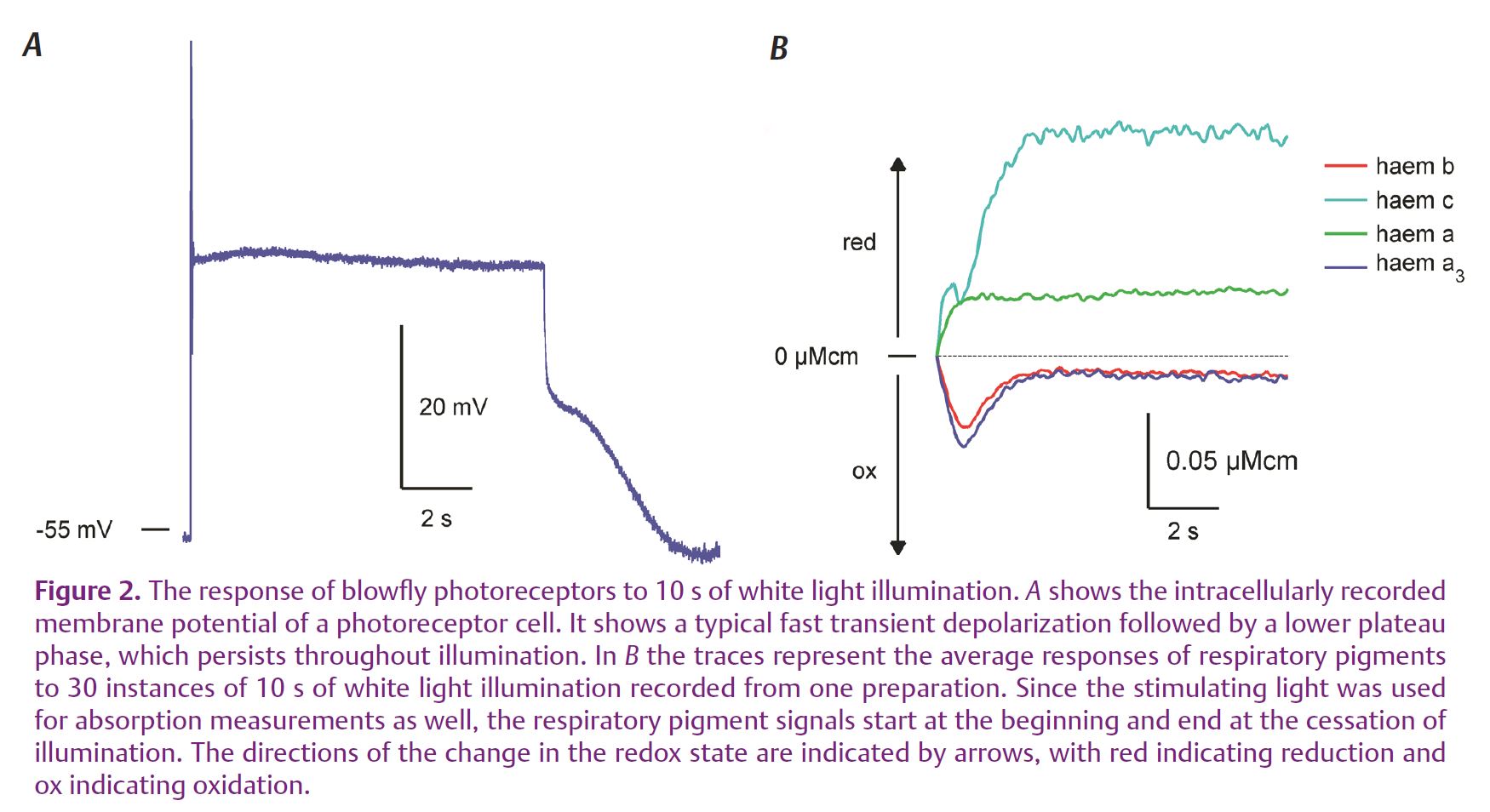
What we discovered was that such a step-like physiological load induces virtually simultaneous changes in the redox state of all cytochromes and these are, in part, very similar to changes of flavoproteins and NADH previously measured using their autofluorescence (Stavenga, 1995). What differs is that some pigments (haems b and a3, as well as NADH and flavoproteins – from NADH and succinate dehydrogenases) undergo a transient oxidation, while others (haems a and c) undergo reduction without a conspicuous transient (Fig. 2). A minor exception is haem c, which seems to show a combination of both responses – possibly due to the fact that we could not optically differentiate between haems c and c1, which is a part of complex III.
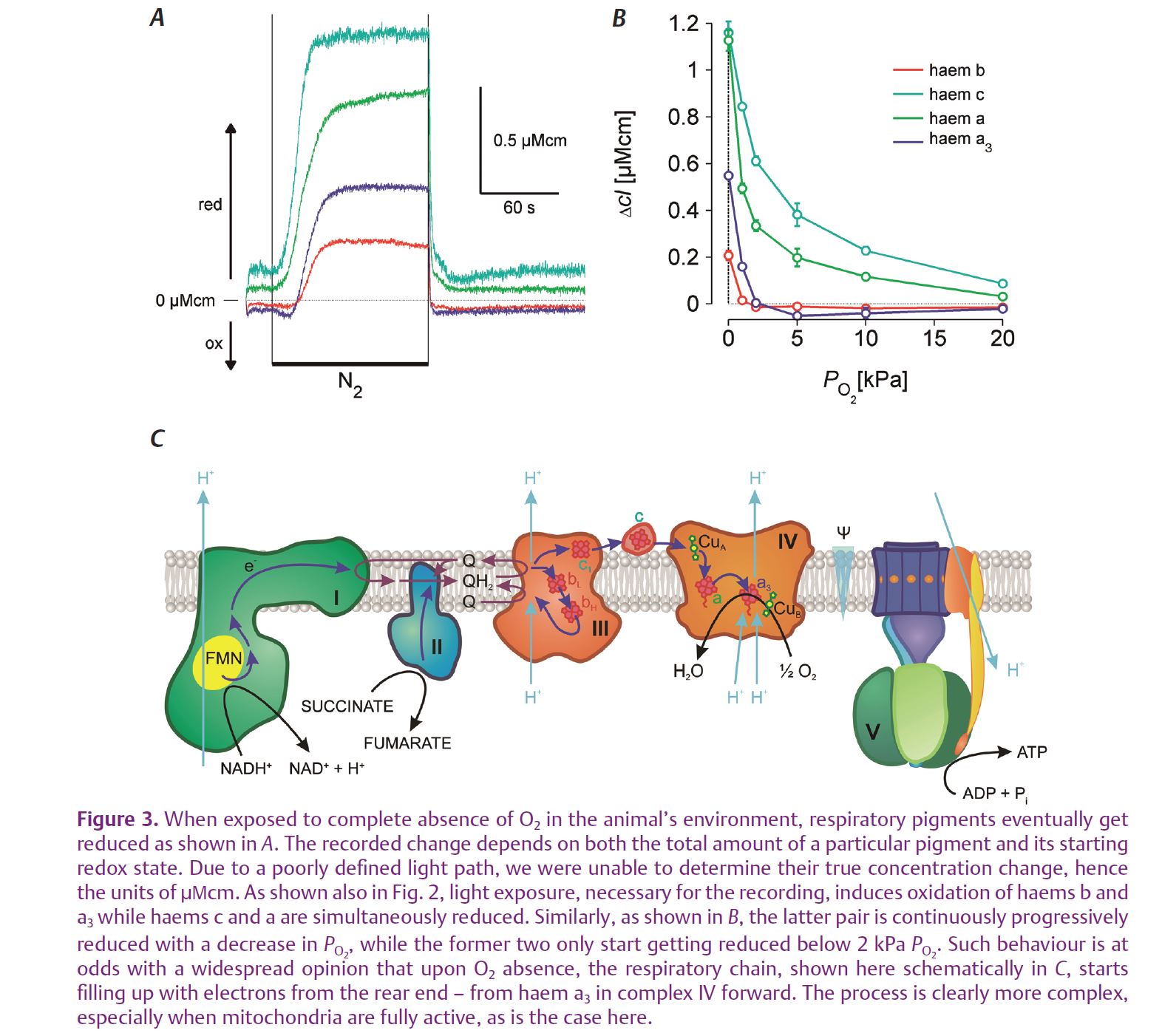
The analysis of responses to hypoxia exposed the same two groups in both steady-state and dynamic PO2 change experiments (Fig. 3). In both cases, haems a and c started to get reduced as soon as the environmental PO2 started to decrease below normal atmospheric level. Haems b and a3, however, first went into oxidation, which only reversed into reduction, due to accumulation of electrons that could not be passed on to O2, below 2 kPa PO2. Of course, this oxidation is most probably due to the fact that the animals were constantly exposed to measuring light and thereby a physiological load. Nevertheless, it clearly shows that the two groups of haems, situated within the same respiratory complexes, behave very differently. This is further illustrated by combining the two sets of data – from hypoxia and illumination experiments (Fig. 4). Especially haems a and a3 – equimolar components of complex IV, the cytochrome c oxidase – have very different steady-state reduction values and they also behave very differently. Haem a3, the more reduced one at rest, exhibits a transient oxidation, while haem a, the highly oxidized one, goes straight into reduction when the photoreceptor cells are illuminated. Although less clear, due to the technical problems of separating haems c from c1 and bH from bL, the story is the same with haems b and c. The likeliest explanation is that this separation of redox states is due to an energy barrier to H+ pumping and electron flow imposed by the inner mitochondrial membrane potential, which is relieved as the mitochondria are activated.
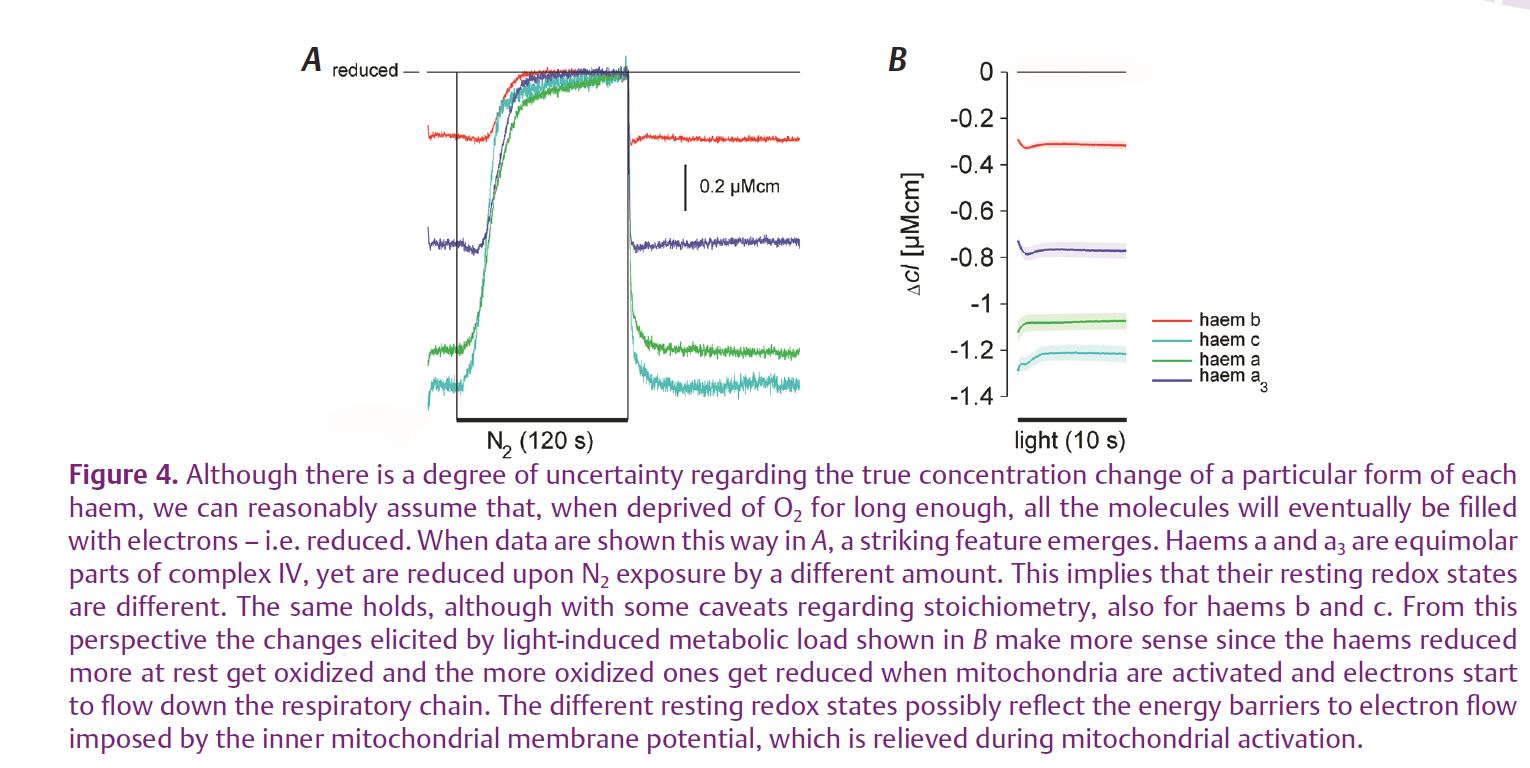
Our results illustrate the fact that, in functioning mitochondria inside a living animal, the conditions are very different to cases where specific protein complexes are studied in great detail in isolation. In the intact mitochondrion within a cell, the particular complexes find themselves in conditions determined simultaneously by many influences like the mitochondrial membrane potential, substrate availability, the respiratory electron flux down the respiratory chain, by the states of electron donor and acceptor molecules, by the interactions with other ions and molecules etc. Many of these are still poorly explored but we believe they are likely to hide important clues to understanding many mitochondrion-related illnesses. We also believe the approach presented here can help in deciphering at least some of them.
References
Chance B & Williams GR (1955). Respiratory enzymes in oxidative phosphorylation II Difference spectra. J Biol Chem 217, 395–407.
Gerster U, Stavenga DG & Backhaus W (1997). Na+/K+ pump activity in photoreceptors of the blowfly Calliphora: a model analysis based on membrane potential measurements. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 180, 113–122.
Hardie RC (2007). TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol 578, 9–24.
Meglič A & Zupančič G (2011). Changes in redox states of respiratory pigments recorded from the eyes of live blowflies exposed to light stimuli and hypoxia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197, 301–310.
Oberwinkler J & Stavenga DG (2000). Calcium imaging demonstrates colocalization of calcium influx and extrusion in fly photoreceptors. Proc Natl Acad Sci U S A 97, 8578–8583.
Stavenga DG (1995). Insect retinal pigments: spectral characteristics and physiological functions. Prog Retin Eye Res 15, 231–259.
Zupančič G (2003). A method for dynamic spectrophotometric measurements in vivo using principal component analysis-based spectral deconvolution. Pflugers Arch 447, 109–119.
