
Physiology News Magazine
Motor neuron activation is conditional during muscle fatigue
The timing of the action potentials discharged by a motor neuron when it is recruited during a fatiguing contraction depends on the amount of synaptic input it requires to reach activation threshold. Action potentials are discharged continuously when the force at which the motor unit is activated is close to the target force of the contraction, but the discharge is much less regular when the target force is further below activation threshold of the motor unit
Features
Motor neuron activation is conditional during muscle fatigue
The timing of the action potentials discharged by a motor neuron when it is recruited during a fatiguing contraction depends on the amount of synaptic input it requires to reach activation threshold. Action potentials are discharged continuously when the force at which the motor unit is activated is close to the target force of the contraction, but the discharge is much less regular when the target force is further below activation threshold of the motor unit
Features
Zachary A Riley & Roger M Enoka
Department of IntegrativePhysiology, University of Colorado, Boulder, CO, USA
https://doi.org/10.36866/pn.73.10

Most fatiguing contractions involve concurrent changes in the force capacity of the activated muscle fibres and in the level of activation of the motor unit pool (Enoka & Duchateau, 2008). Simplistically, one might expect the rate of increase in motor unit activity to match the rate of decrease in force capacity so that the muscle can sustain the force required for the task. Such a relation is not observed, however, because the properties of the motor neurons and the synaptic inputs they receive change during a fatiguing contraction. Consequently, activation of the motor unit pool must accommodate decreases in both the force capacity of the muscle fibres and the output discharged by the motor neurons.
When an individual sustains a muscle contraction at a submaximal intensity, motor units that were activated at the start of the task usually exhibit a decrease in the rate at which they discharge action potentials. To maintain the target force, the nervous system responds to decreases in discharge rate by increasing the synaptic input to the motor neuron pool. Although the increase in excitation does not prevent the decrease in discharge rate, it does activate previously quiescent motor units provided the target force is less than the upper limit of motor unit recruitment (Mottram et al. 2005). The discharge characteristics of the newly recruited motor units, however, indicate that the synaptic input received by the motor neuron pool during the fatiguing contraction involves more than a gradual increase in excitation.
Discharge variability
When a motor neuron is activated by the injection of a depolarizing current, the response is dictated by the intrinsic properties of the neuron and the timing of action potentials elicited by the input is more regular than that observed during activity evoked by synaptic input (Berg et al. 2007; Matthews, 1996). The regularity of the discharge is often expressed as the normalized variability (coefficient of variation) of the duration between consecutive action potentials, which are known as interspike intervals. When a series of brief isometric contractions are performed around the recruitment threshold of a motor unit, the interspike intervals vary across the target forces. In addition to the expected change in mean interspike interval with variation in target force, there is a change in the variability in the timing of the action potentials. The coefficient of variation for interspike interval is greatest at a target force just above the recruitment threshold of the motor unit (~30%), and then it decreases to a steady-state value (~10%) with increases in the target force (Barry et al. 2007; Matthews, 1996).
A motor neuron will discharge an action potential when its membrane potential is depolarized to a value that exceeds the voltage threshold for the generation of an action potential. When a motor neuron is activated during a voluntary contraction and discharges a series of action potentials, the generation of each action potential depends on the interaction between the recovery of the membrane potential from the preceding action potential and the fluctuations in membrane potential due to the continuous synaptic input received by the neuron. The influence of the synaptic input, which comprises both excitation and inhibition, on the variation in membrane potential is characterized as synaptic noise. At low levels of activation, the greater variability in discharge times is attributed to action potentials being generated by transient depolarizations of membrane potential associated with synaptic noise (Matthews, 1996).
Discharge during fatiguing contractions
Motor units that are recruited at the onset of a fatiguing contraction typically experience a gradual decrease in discharge rate that is attributed to the combined effects of motor neuron adaptation and an increase in inhibitory synaptic input (Mottram et al. 2005). Although the progressive increase in net excitation of the motor neuron pool recruits additional motor units, the discharge characteristics suggest a significant involvement of synaptic noise in the generation of the action potentials. Riley et al. (2008) recorded the discharge of single motor units in biceps brachii as participants sustained an isometric contraction with the elbow flexor muscles at a target force that was less than the recruitment threshold of the motor unit. The contraction was sustained until the motor unit was recruited and discharged action potentials for several minutes. Two discharge patterns were observed: the newly recruited motor unit discharged action potentials either continuously or intermittently (Fig. 1). No motor unit exhibited both patterns during the same contraction, but individual motor units could produce both patterns in different contractions when the target force was varied.
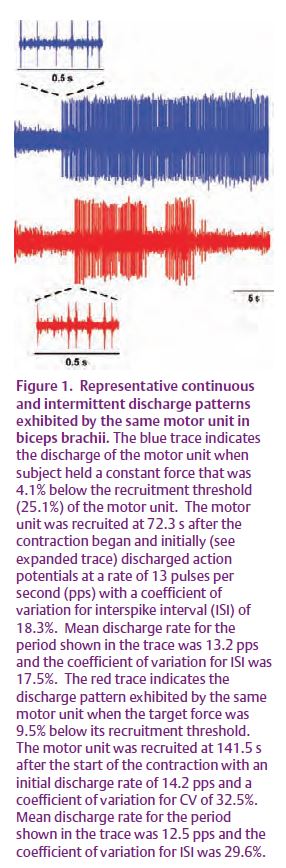
Expression of the two patterns was related to the difference between the target force and the recruitment threshold of the motor unit. The discharge was continuous when the target force was close to recruitment threshold (~5% of maximal voluntary contraction [MVC] force), whereas it was intermittent for greater differences (~10% MVC force) between the two forces. Mean discharge rate and the variability of discharge times differed for the two patterns and from those recorded during a standardized gradual increase in muscle force when recruitment threshold was assessed.
Mean discharge rate at recruitment during the fatiguing contraction was greater and the coefficient of variation for interspike interval was lower for the continuous pattern compared with the intermittent pattern. Mean discharge rate during the sustained contraction declined during the continuous pattern but not the intermittent pattern, and the coefficient of variation remained greater for the intermittent pattern during the contraction.
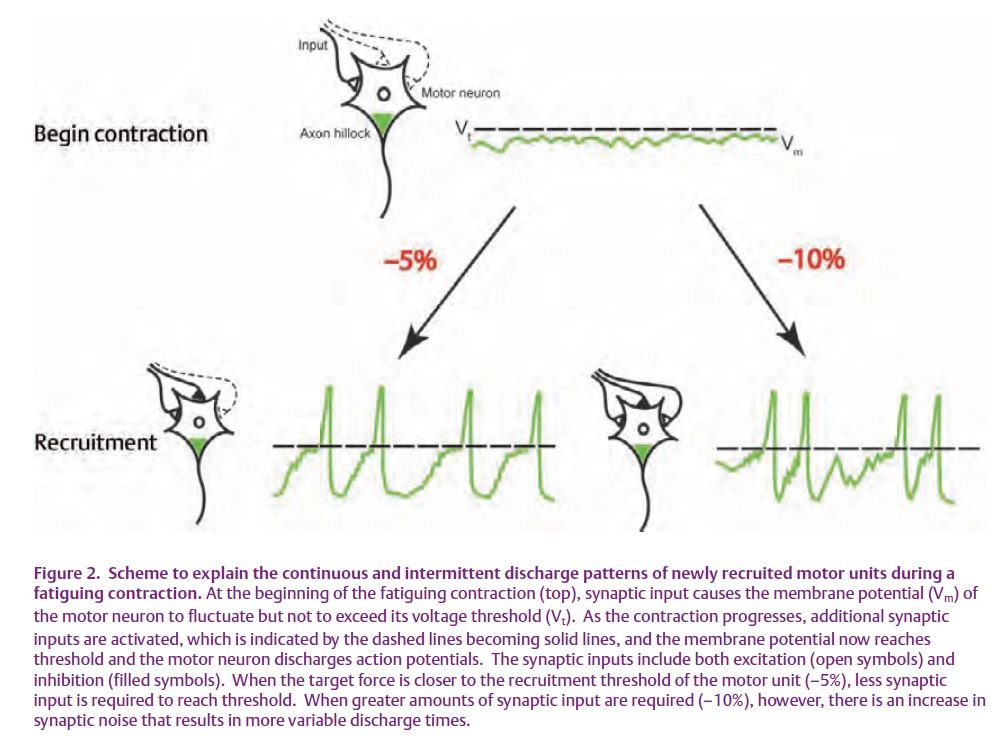
Because the intermittent discharge of action potentials occurred when there was a greater difference between the target force and recruitment threshold, the greater variability in discharge during this condition, at least at recruitment during the fatiguing contraction, might be explained by an augment-ation of synaptic noise (Fig. 2). However, why the discharge variability remained elevated during the intermittent pattern and even why the discharge stopped for several seconds during the sustained contraction is more difficult to explain. Presumably, the greater net excitation required to reach threshold when the target force was further from recruitment threshold was accompanied by an increase in inhibitory synaptic input that produced a less regular discharge pattern (Berg et al. 2007).
Acknowledgements
This work was supported by an award to RME from the National Institute of Neurological Disorders and Stroke in the USA.
References
Barry BK, Pascoe MA, Jesunathadas M & Enoka RM (2007). Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97, 3206-3218.
Berg RW, Alaburda A & Hounsgaard J (2007). Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315, 390-393.
Enoka RM & Duchateau J (2008). Muscle fatigue: what, why and how it influences muscle function. J Physiol 586, 11-23.
Matthews PB (1996). Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492, 597-628.
Mottram CJ, Jakobi JM, Semmler JG & Enoka RM (2005). Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93, 1381-1392.
Riley ZA. Maerz AH, Litzey JC, Enoka RM (2008). Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586, 2183-2193.
