
Physiology News Magazine
Multimerisation – an only transporter is a lonely transporter
Features
Multimerisation – an only transporter is a lonely transporter
Features
David Meredith & Richard Boyd
Department of Physiology, Anatomy & Genetics, University of Oxford, South Parks Road, Oxford, UK
https://doi.org/10.36866/pn.62.20

The Oxford English Dictionary definition of a multimer is ‘An aggregate of molecules held together by relatively weak bonds, such as hydrogen bonds’.
As all biological systems include water, by this definition all proteins would be multimers – hence the need for a more physiologically relevant definition. This would not be confined to a molecular aggregate held together by weak bonds, but would include ‘salt bridges’ and covalent bonds; but critically in this new definition the aggregation must be between protein molecules.
A monomer transport protein is a single polypeptide chain that possesses the intrinsic capability of transporting its substrate(s) and additionally exists in the membrane without interacting with other proteins (Fig. 1A).
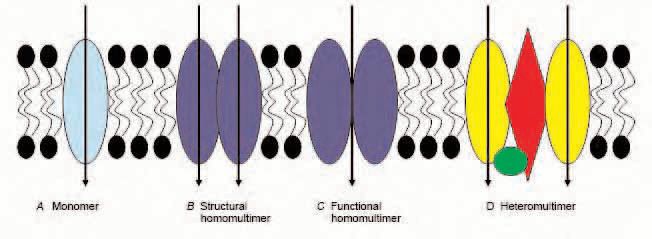
It is surprisingly difficult to find examples of membrane transport proteins cited in the literature as being shown to be monomers. One example is the bacterial lactose transporter, lac permease (reviewed in Kaback, 2005); another is the mammalian sodium/glucose cotransporter SGLT1, shown by Eskandari et al. (1998), with freeze fracture electron microscopy allowing measurement of the particle size of the cloned transporter expressed in Xenopus oocytes. However, even these two examples are not unchallenged: the lac permease has been suggested to be a dimer by a number of researchers, with the experimental conditions (such as the presence or absence of a physiological electrochemical gradient (Golkorn et al. 1984), or the detergent used (Houssin et al. 1984) being proposed to affect the subunit interactions. Earlier reports from radiation inactivation experiments are consistent with proposals that SGLT1 exists as a multimer (Lin et al. 1984), while a decade later Koepsell & Spangenberg (1994) reported that ‘the functional Na+-glucose cotransport systems in mammals are composed of two SGLT1-type subunits’ (as well as a regulatory protein) using similar techniques.
A heteromultimer is a multimer (as defined above) where the subunits are not all identical. Thus it could either consist of a number of (all) different subunits/ polypeptides, or of a number of identical subunits plus one or more other distinct protein.
The topic of heteromultimeric transport proteins is too large to be given more than the briefest treatment here, but includes:
- proteins which modify the function of others e.g. the NHERF family (reviewed by Shenolikar et al. 2004);
- true multi-subunit transport proteins (e.g. amino acid transporters, reviewed by Verrey et al. 2004); or
- proteins required (at least) for expression (e.g. MCT1 and CD147, reviewed by Halestrap & Meredith, 2004).
There are two ways of defining multimers that contain two or more identical transporter polypeptides, based on the way the identical subunits interact, irrespective of whether they are in a heteromultimer or a homomultimer (where all the subunits are identical polypeptide sequences).
In a structural multimer each transporter protein subunit has the intrinsic capability of transporting its substrate, but in the membrane the protein is found associated with (at least) other polypeptides of sequence identical to that of the subunit (Fig. 2B).

For example, each individual subunit of SGLT1 is thought to have its own substrate binding site and translocation pathway, based on the finding that SGLT1 transports glucose when expressed in Xenopus oocytes yet appears as monomers in freeze-fracture electron microscopy. Thus it would appear that if/when the proteins are present as a dimer, this is because they are physically more stable in this form.
While at the present time mammalian transport proteins are virtually impossible to crystallise, more progress has been made with those from bacteria. For example, the bacterial multidrug resistance transporter EmrE has been shown to be an asymmetric homodimer (Ubarretxena-Belandia & Tate 2004), with each subunit crystallised with a tetraphenylphosphonium ion (TPP+) substrate bound to it. As the binding sites were virtually central to the protein, it would be logical to speculate that each subunit might act as a transporter if in the (albeit unphysiological) monomeric state.
In a functional multimer each transporter protein subunit does not have the intrinsic capability of transporting its substrate, but has to be associated with (at least) other polypeptides of sequence identical to that of the subunit in order to function (Fig 2C, D).
The ability of a mutant subunit of a transporter multimer to inhibit the activity of a wildtype one indicates that the interaction between the subunits in the multimer is essential for the functioning of the individual subunits, that is, the individual subunits do not have intrinsic transport capability. Thus the mutant protein exerts a dominant negative effect on the wildtype proteins, and this can be measured by a drop in activity when the mutant and wildtype proteins are co-expressed in a heterologous expression system such as Xenopus oocytes. There are a number of explanations for why a dominant negative phenomenon might be observed, e.g. the binding site is made up of parts of a number of subunits, or a single subunit protein does not have the correct shape to be functional.
Analysis of the reduction of transport when known ratios of mutant and wildtype protein are expressed, with an assumption of a binomial distribution of mutant and wildtype protein complexes, allows modelling of the data to estimate the number of subunits in the multimer. This approach was used by Casula et al. (2001) for the KCC1 K-Cl cotransporter, and in our laboratory for the proton-coupled peptide transporter PepT1 (Panitsas et al. 2006). Data from such an experiment on PepT1 is shown in Fig. 2A. The model that best fits the data (shown by the solid line) represents a tetramer, with at least two wildtype subunits (Fig. 2B). One conclusion would be that the mutant PepT1 shows a dominant negative effect, and that the tetramer actually consists of a dimer of functional dimers (Fig. 2C).
Why is it important to know whether a transport protein is a monomer or a multimer, and, if the latter, of what type? There is a paucity of high resolution structures for membrane transporters, and computer modelling is one way of providing a working model. Therefore, if the protein of interest is for example a functional multimer, this will be crucial information. In a very recent paper by Jen et al. (2005), a heterozygous mutation was identified in a neurotransmitter reuptake system for glutamate (EAAT1) in a patient with episodic ataxia, seizures, migraine and alternating hemiplegia. When the mutant EAAT1 was expressed with the wildtype (as would happen in a heterozygous individual) the function of the wildtype was severely compromised. The decreased glutamate uptake is predicted to result in the neuronal hyperexcitability and the symptoms reported.
Therefore, multimerisation is not just of academic but also of clinical importance.
Acknowledgement
The authors are grateful to the Wellcome Trust for supporting their work.
References
Casula S, Shmukler BE, Wilhelm S, Stuart-Tilley AK, Su W, Chernova MN, Brugnara C & Alper SL (2001). A dominant negative mutant of the KCCl K-Cl cotransporter: both N- and C-terminal cytoplasmic domains are required for K-Cl cotransport activity. J Biol Chem 276, 41870-41878.
Eskandari S, Wright EM, Kreman M, Starace DM & Zampighi GA (1998). Structural analysis of cloned plasma membrane proteins by freeze-fracture electron microscopy. Proc Natl Acad Sci USA 95, 11235-11240.
Goldkorn T, Rimon G, Kempner ES & Kaback HR (1984). Functional molecular weight of the lac carrier protein from Escherichia coli as studied by radiation. inactivation analysis. Proc Natl Acad Sci USA 81, 1021-1025.
Halestrap AP & Meredith D (2004). The SLC16 gene family from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447, 619-628.
Houssin C, le Maire M, Aggerbeck LP & Shechter E (1985). The lactose permease of Escherichia coli: evidence in favor of a dimer. Arch Biochem Biophys 240, 593-606.
Jen JC, Wan J, Palos TP, Howard BD & Baloh RW (2005). Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia and seizures. Neurology 65, 529-534.
Kaback HR (2005). Structure and mechanism of the lactose permease. C R Biol 328, 557-567.
Koepsell H & Spangenberg J (1994). Function and presumed molecular structure of Na+-D-glucose cotransport systems. J Memb Biol 138, 1-11.
Lin JT, Szwarc K, Kinne R & Jung CY (1984). Structural state of the Na+-D-glucose cotransporter in calf kidney brush-border membranes. Target size analysis of Na+- dependent phlorizin binding and Na+dependent D-glucose transport. Biochim Biophys Acta 777, 201-208.
Panitsas KE, Boyd CAR & Meredith D (2006). Evidence that the rabbit proton-peptide co-transporter PepT1 is a multimer when expressed in Xenopus laevis oocytes. Pflugers Arch (in press).
Shenolikar S, Voltz JW, Cunningham R & Weinman EJ (2004). Regulation of ion transport by the NHERF family of PDZ proteins. Physiology 19, 362-369.
Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y (2004). CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 447, 532-542.
Ubarretxena-Belandia I & Tate CG (2004). New insights into the structure and oligomeric state of the bacterial multidrug transporter EmrE: an unusual asymmetric homo-dimer. FEBS Lett 564, 234-238.
