Physiology News Magazine
Muscle’s silent sensors
This is a story about measurement of position sense at the elbow joint. If I cannot see my arm, where is it? The object of our studies has been to understand the changes in position sense seen after exercise and during loading of the arm. Our choice of the elbow joint is simply out of convenience. Some recent experiments using the knee joint and the quadriceps muscle have shown that the principles we have considered at the elbow joint also applies at other joints.
Features
Muscle’s silent sensors
This is a story about measurement of position sense at the elbow joint. If I cannot see my arm, where is it? The object of our studies has been to understand the changes in position sense seen after exercise and during loading of the arm. Our choice of the elbow joint is simply out of convenience. Some recent experiments using the knee joint and the quadriceps muscle have shown that the principles we have considered at the elbow joint also applies at other joints.
Features
Uwe Proske & Trevor Allen
Department of Physiology, Monash University, Clayton, Victoria, Australia
https://doi.org/10.36866/pn.69.22
We have been using a property of muscle, called thixotropy, as a tool for investigating position sense (Physiology News 66, 20–22). Another word for thixotropy is muscle history-dependence. This is a method which allows us, at a given muscle length, to change the levels of background activity in muscle spindles of elbow muscles by contracting them while they are held short or long and then returning them to the initial length. Muscle spindles are believed to be largely responsible for signalling to the brain muscle length and therefore elbow angle. The changes in background activity following conditioning lead subjects to think that muscle length has changed and so they make errors in limb position sense.
The way we have been doing the experiments is to use a position matching task. The experimenter places one forearm (reference) at a given angle and the subject is asked to match its position by placement of their other arm (indicator). One fact that emerged in recent experiments (Allen et al. 2007) is that it is necessary to consider the muscle history-dependence of both reference and indicator arms.
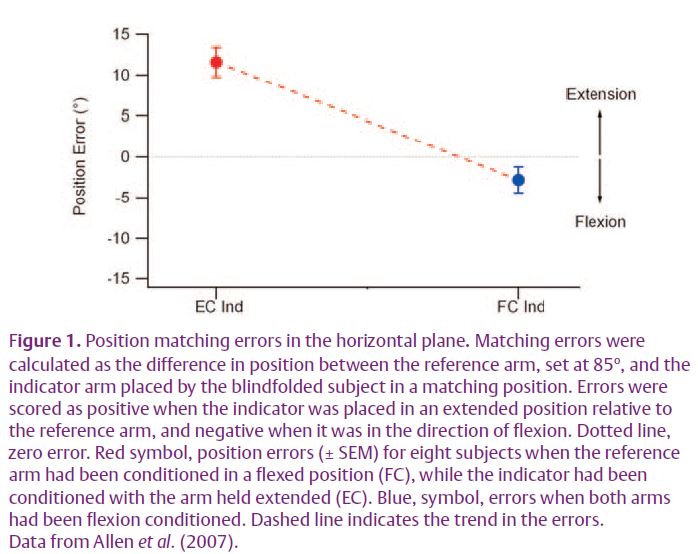
In the recent experiments we began to systematically control for muscle history effects in both arms. That has revealed an unexpected distribution of position errors. For these experiments, to avoid the effects of gravity, measurements were made in the horizontal plane. First, when contraction history in both arms was made the same, unexpectedly, position errors did not reduce to zero. So, for example, in Fig. 1 when both arms were conditioned by contracting elbow flexors with the arms held flexed (flexion conditioning), the subjects consistently placed their indicator arm in a position that was slightly more flexed (blue symbol) than the position adopted by the reference arm (dotted line). A second observation made was that when the reference arm was again flexion conditioned, but this time the indicator arm had its elbow extensors contracted with the arm held extended (extension conditioning), the subject thought that their reference arm was much more extended that it really was (red symbol). In other words, by simply changing the form of conditioning of the indicator arm, subjects believed that arm position had shifted by over 14o. These errors can be satisfactorily explained by changes in background activity of muscle spindles of both arms, as a result of the thixotropic property of their intrafusal fibres (Allen et al. 2007). That conclusion led us to pay a lot more attention to the state of conditioning of the two arms.
An issue that has fascinated me for a long time is the question, how can muscle spindles act as position sensors during muscle contraction? Muscle spindles, as stretch receptors, are able to signal the length of a muscle. At the same time, because they have an inbuilt sensitivity control, the fusimotor system, they are able to change their discharge without any accompanying length change. The problem was, how did the central nervous system distinguish between activity arising from muscle length changes and from intrafusal contractions?
The current accepted view is based on a model put forward by McCloskey et al. (1983). The idea is that whenever the central nervous system generates activity in fusimotor neurons this is subtracted from the gross spindle discharge, to leave only the length-related signal to reach consciousness. Spindles are activated through the fusimotor system whenever we carry out a voluntary contraction.
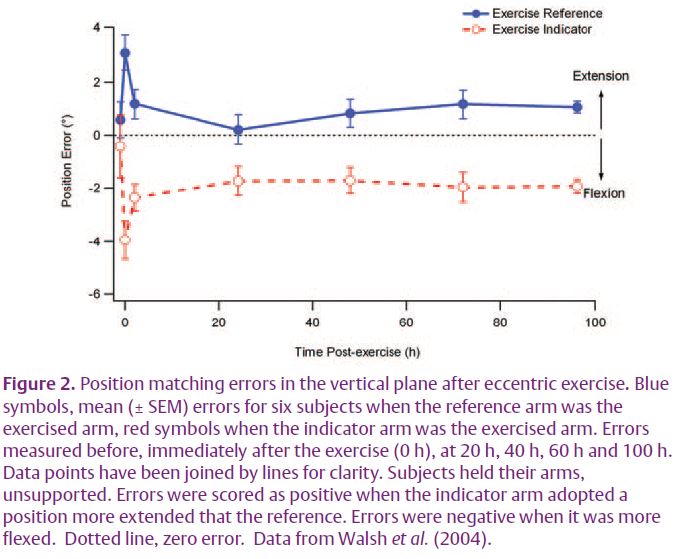
There are reasons for thinking that what is going on is more complicated than that. Here we were guided by some experiments on the effects of eccentric exercise on position sense (Walsh et al. 2004). These experiments involved a position matching task in the vertical plane. After eccentric exercise of one arm this was weakened as a result of the muscle damage and fatigue following the exercise (Proske & Morgan, 2001). When the exercised arm was the indicator, the subject made significant matching errors, placing it in a position that was more flexed than the position adopted by the reference arm (Fig. 2). Conversely, when the reference arm was fatigued matching errors lay in the direction of extension. To explain the result we proposed that when an arm was fatigued, it required more effort to hold it in a given position and the effort signal provided positional information. When the indicator was fatigued, the greater effort required to support its weight was interpreted as the arm being in a more extended position, where the gravity vector acting on it was greater. So in making a match, subjects flexed their indicator to a point where the effort signals in the two arms matched, leading to errors in the direction of flexion. Predictably, errors reversed when it was the reference arm that had been exercised (Fig. 2). So we were proposing that during a muscle contraction the errors in position sense arose from a new position signal derived from a centrally generated sense of effort.
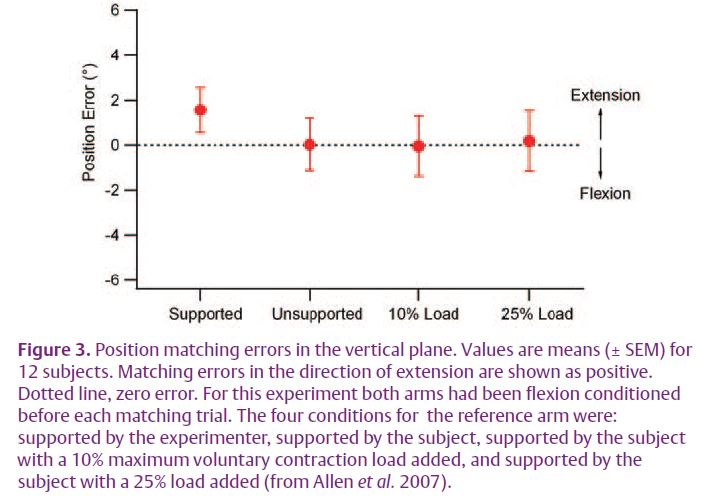
A simple and direct test of the effort hypothesis was to examine the effects of loading the arm by the addition of weights. Increasing the load should increase the effort required to support the arm and therefore produce position errors. In this new experiment we were careful to make sure from the outset that for each trial both arms had been conditioned identically. Then, when one arm was loaded during the match, position errors seen previously (Winter et al. 2005) were no longer present (Fig. 3).
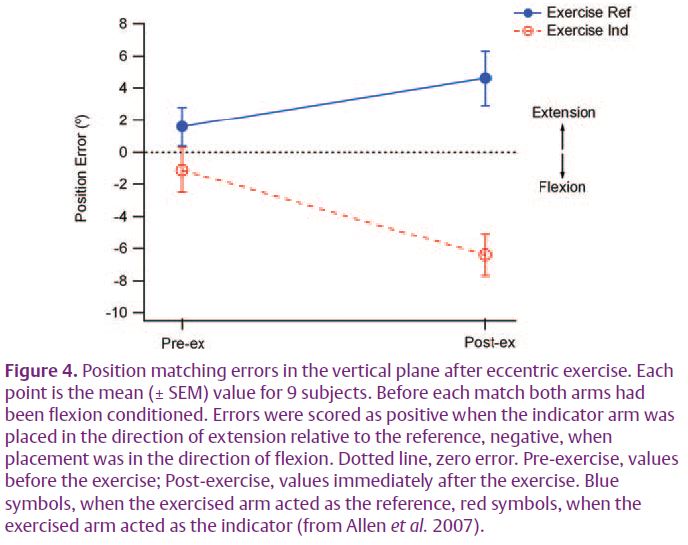
Presumably in the previous study the observed errors had been thixotropyrelated. Subsequently we repeated the experiment on eccentric exercise, again making sure that both arms had first been identically conditioned. (Fig. 4). When the reference arm had been exercised, error lay in the direction of extension. When the indicator was exercised they lay in the direction of flexion (Allen et al. 2007). This was the same pattern as reported by Walsh et al. (2004), but now we were no longer invoking the sense of effort as responsible for the errors. If effort had played a significant role it should have shown up in the experiments of loading the arm, which it did not.
How do we explain all of this? Sticking to the simple result of Fig. 3, the conclusion is reached that in the absence of vision we are able to locate, equally accurately, the position of our arms, whether they are loaded or not, provided that beforehand they have been conditioned identically. So, by inference, whether arm muscles are contracting or not does not alter our sense of forearm position. Given that during voluntary contractions the fusimotor neurons are co-activated, the result could be interpreted as evidence in support of the operation of the McCloskey et al. (1983) subtraction model.
But there were bits of evidence which did not quite fit such an interpretation. Experiments on the paralysed, anaesthetised arm had shown that a large position signal of central origin was available when peripheral afferent activity had been blocked (Gandevia et al. 2006). Presumably with such a block in place, the command signals for fusimotor co-activation during attempted movements would still be present and any subtraction from an absent afferent signal might have been expected to lead to a negative outcome, perceived movements in a direction opposite to that which had been willed (Gandevia et al. 1993). That was not the case. It led us to consider another explanation and here we were attracted by propositions involving an internal forward model (Bays & Wolpert, 2007).
The idea is simple enough. As soon as a voluntary contraction of arm muscles accompanies the movement, or maintained position, an efference copy of the motor command signal to the muscle becomes available. The efference copy accesses memory stores of similar movements carried out in the past to compare the afferent feedback that had accompanied those movements with the feedback during the present movement. Only the difference signal reaches consciousness. When the anticipated and fed-back signals are the same, the posture is confirmed with no accompanying sensation of a change in position, thus the label of muscle receptors as ‘silent sensors’.
Finally, it is necessary to explain the effects of exercise. We have now done exercise experiments on both arm muscles and leg muscles. The interpretation that is consistent with all of the observations is that we perceive an exercised muscle as longer than it actually is. For the arm, when the elbow flexors have been exercised, the arm is perceived as more extended (longer flexors) than is really the case. Our interpretation, in terms of the operation of an internal forward model, is that the afferent feedback for maintenance of a given position is higher, as a result of fatigue, and that is interpreted as a longer muscle.
We are now on the lookout for experiments that will test the forward model hypothesis more directly. What happens when the muscle is paralysed, that is, afferent feedback is present, but deprived of fusimotor activation? Will there be negative effects this time? Whatever the outcome, there are two important lessons from all of this. One is that the effects of muscle thixotropy on muscle spindle discharges can lead to misinterpretation of observations on limb position sense, and secondly, we should not underestimate the level of sophistication involved in the central processing of proprioceptive information.
References
Allen TJ, Ansems GE & Proske U (2007). Effects of muscle conditioning on position sense at the human forearm during loading or fatigue of elbow flexors and the role of the sense of effort. J Physiol 580.2, 423–434.
Bays PM & Wolpert DM (2007). Computational principles of sensorimotor control that minimize uncertainty and variability. J Physiol 578, 387–396.
Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB & Hales JP (1993). Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol 470, 85–107.
Gandevia SC, Smith JL, Crawford M, Proske U & Taylor JL (2006). Motor commands contribute to human position sense. J Physiol 571, 703–710.
McCloskey DI, Gandevia SC, Potter EK & Colebatch JG (1983). Muscle sense and effort: motor commands and judgements about muscular contractions. In Motor Control Mechanisms in Health and Disease, ed. Desmedt JE, pp. 151–167. Raven Press, New York.
Proske U & Morgan DL (2001). Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537, 333–345.
Walsh LD, Hesse CW, Morgan DL & Proske U (2004). Human forearm position sense after fatigue of elbow flexor muscles. J Physiol 558, 705–715.
Winter JA, Allen TJ & Proske U (2005). Muscle spindle signals combine with the sense of effort to indicate limb position. J Physiol 568, 1035–1046.