Physiology News Magazine
Muscles under stress
It is often assumed that skeletal muscles are not among the targets of the sympathetic nervous system (SNS). However, muscles receive abundant (nor)adrenergic innervation, mostly but not exclusively addressed to arterioles, and are affected by the hormone adrenaline, released by the adrenal medulla (under sympathetic control). In fact, catecholamines modulate many functions of skeletal muscle fibres, ranging from excitability to contractility to metabolism
Features
Muscles under stress
It is often assumed that skeletal muscles are not among the targets of the sympathetic nervous system (SNS). However, muscles receive abundant (nor)adrenergic innervation, mostly but not exclusively addressed to arterioles, and are affected by the hormone adrenaline, released by the adrenal medulla (under sympathetic control). In fact, catecholamines modulate many functions of skeletal muscle fibres, ranging from excitability to contractility to metabolism
Features
Silvestro Roatta (1) & Dario Farina (2)
1: Dipartimento di Neuroscienze, Sezione di Fisiologia, Università di Torino, Torino, Italy
2: Center for Sensory-Motor Interaction (SMI), Department of Health Science and Technology, Aalborg University, Aalborg, Denmark
https://doi.org/10.36866/pn.75.15


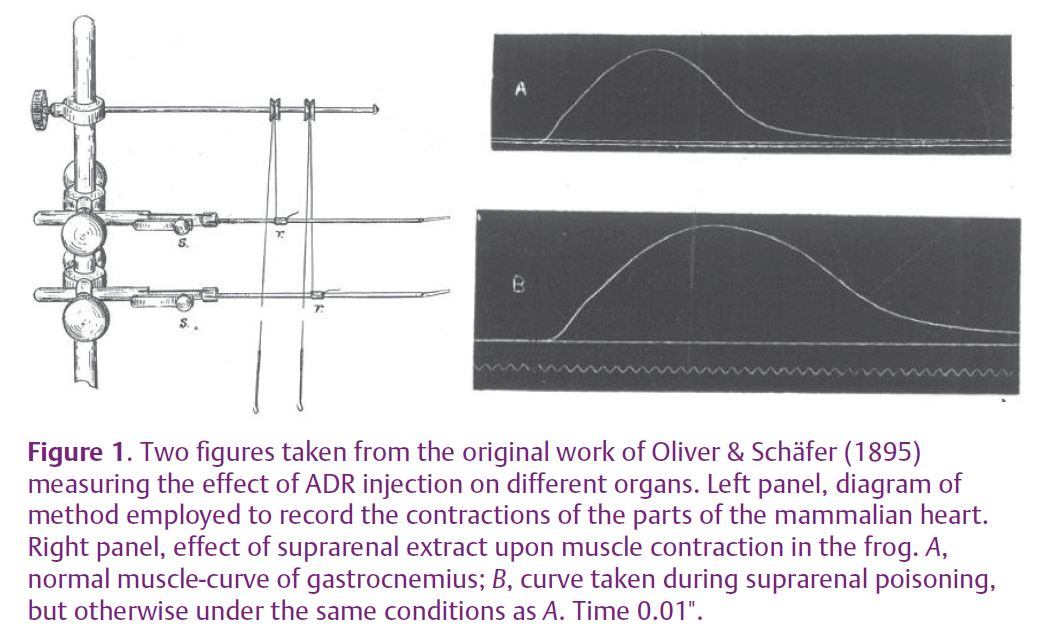
The first evidence of adrenergic modulation of muscle function dates back to the end of the 19th century, before the biochemical identification of catecholamines. At that time, scientists investigated the function of the adrenal gland by systemic administration of ‘suprarenal extracts’ in experimental animals. The results were puzzling as the animals exhibited reactions leading to death with different time courses and symptoms, so that the gland was believed to be a poison collector. Oliver & Schäfer (1895) first described specific effects of suprarenal extract on skeletal muscle as well as on the heart and other organs and observed increased amplitude and prolonged duration of the twitch force of the frog gastrocnemius muscle (Fig. 1).
Since then, the issue has been investigated extensively, and catecholamines are now known to modulate several processes in skeletal muscle fibres (Bowman, 1980; Roatta & Passatore, 2008), particularly through β (mainly β2) adrenergic receptors. Most of these actions are functionally meaningful in the context of a ‘fight-or-flight’ reaction, i.e. the state of alertness generally provoked by an impending threat or danger. This condition is characterized by a generalized activation of the sympathetic nervous system (SNS) that is aimed at supporting the intense motor activity necessary to face or to escape from the threat; this occurs in animals as well as humans. Although in the latter the motor component is often suppressed by social constraints, the autonomic activation still occurs under stressful conditions, with several side effects. In addition, it is worth emphasizing that circulating adrenaline (ADR), rather than neurally released noradrenaline, is the main mediator of the sympathetic action on skeletal muscles, given the much higher affinity of ADR for the β2 adrenergic receptors. This is important from the functional point of view, since there is a different release of the two substances depending on the context and on the stressor. For instance, ADR release is stimulated by painful stimuli and during panic attacks but not by cold exposure.
Catecholamines exert an anti-fatigue action on muscle fibres. By potentiating the action of the Na+-K+ pump they help in counteracting the decrease of ionic gradients across the cell membrane that occurs under intense activity and that impairs the excitability of muscle fibres, thus contributing to fatigue.
Moreover, by acting presynaptically at the motor end-plate, catecholamines potentiate the neuromuscular transmission. This action does not have a functional consequence in normal conditions, given the high reliability of the motor end-plate in transmitting excitation to the muscle fibre. However, in conditions in which the fibre excitability is impaired (e.g. during intense muscle activity), this effect may be useful.
Catecholamines increase the availability of energy substrates by promoting glycogenolysis and glucose absorption. β2 agonists also affect protein metabolism by slowing proteolytic processes and by potentiating protein synthesis. They are indeed employed as anabolic drugs in the livestock industry and in certain power-related sports, e.g. weight lifting and bodybuilding.

Finally, going back to the initial observation of Oliver and Schäfer, catecholamines modulate the contractility of muscle fibres (inotropic effect). In his extensive review, Bowman provided evidence that the adrenergic action depends on the muscle fibre type: the twitch force is increased in amplitude
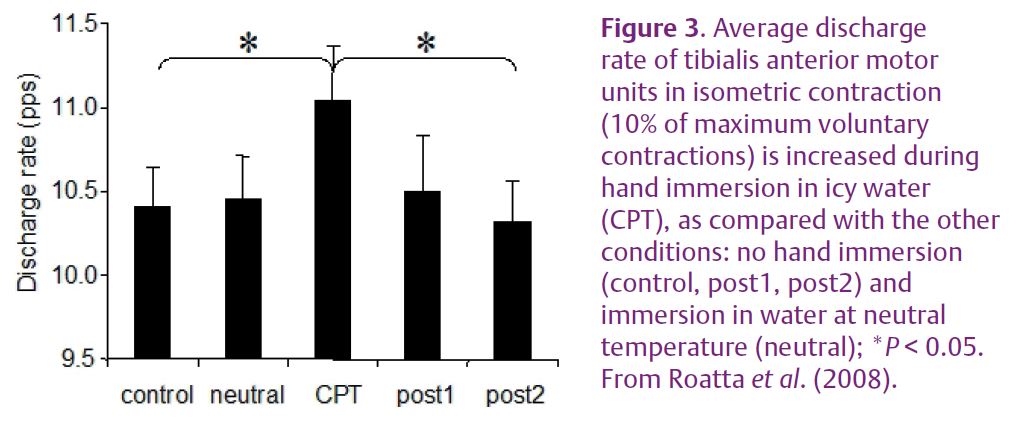
and duration in fast-twitch muscle fibres whereas the opposite effect is exhibited by slow-twitch fibres (Bowman, 1980) (Fig. 2). More recent in vitro studies elucidated the mechanisms underlying these effects. The twitch potentiation is mediated by enhanced Ca2+ release from the sarcoplasmatic reticulum, and this mechanism is shared by both fibre types. The twitch decrease and shortening is instead related to an increased rate of Ca2+ re-uptake into the sarcoplasmatic reticulum, an effect mediated by phospholamban, a regulatory protein controlling the activity of Ca2+ pumps (Ha et al. 1999). In a similar way, catecholamines increase the relaxation rate of the heart (positive lusitropic effect). This effect is not exhibited by fast twitch muscle fibres because they lack phospholamban, whereas it may be responsible for a marked weakening of slow-twitch fibres (Fig. 2D), as documented in many animal experiments and in vitro studies. We recently investigated whether this phenomenon is functionally relevant by analysing it during voluntary contractions and physiological activation of the SNS in humans (Roatta et al. 2008). In this study, healthy men were subjected to the cold pressor test (CPT, a sympathetic activation test based on the painful immersion of a hand in icy water) while the activity of low-threshold (presumably slow-twitch) motor units was recorded from the tibialis anterior muscle during isometric ankle dorsi-flexion. The experiments showed that, as compared to control conditions, the discharge rates of the recruited motor units increased in order to attain the same force level (10% of the maximum voluntary contraction) during CPT (Fig. 3). This observation indicated a weakening effect of SNS activation on the investigated muscle. In addition, the twitches of single motor units, estimated by the spike-triggered averaging technique, exhibited an increased relaxation rate during CPT, confirming the observations in animal experiments. Although these data need to be confirmed and substantiated by other experiments, they indicate that the influence of the SNS on skeletal muscle is not negligible as it can produce appreciable changes in muscle function and motor control.
One may wonder how a ‘weakening’ effect could fit with the ‘fight-or-flight’ reaction where more muscle power would be expected. It should be noted, however, that (1) the big, fast-twitch motor units are not affected by this action; and (2) the maximal (tetanic) force of slow-twitch motor units is not impaired by this effect, although a higher discharge rate will be required to attain it.
Moreover, a faster relaxation rate of slow muscle fibres may be useful in the fast movements required in the ‘flight’. In this respect, it is interesting that chronic administration of β2 agonists produces a transformation of type-I (slow-twitch) to type-II (fast-twitch) in muscle fibres.
On the other hand, some of these actions may be harmful to the muscles under stress-related adrenergic overload, and it has been hypothesized that they might be implicated in the development of musculoskeletal disorders.
References
Bowman WC (1980). Effects of arenergic activators and inhibitors on skeletal muscles. In Handbook of Experimental Pharmacology, Adrenergic Activators and Inhibitors, ed. Szekeres L, pp. 47–128. Springer, Berlin, Heidelberg, New York.
Ha TN, Posterino GS & Fryer MW (1999). Effects of terbutaline on force and intracellular calcium in slow-twitch skeletal muscle fibres of the rat. Br J Pharmacol 126, 1717–1724.
Oliver G & Schäfer EA (1895). The physiological effects of extracts of the suprarenal capsules. J Physiol 18, 230–276.
Roatta S, Arendt-Nielsen L & Farina D (2008). Sympathetic-induced changes in discharge rate and spike-triggered average twitch torque of low-threshold motor units in humans. J Physiol 586, 5561–5574.
Roatta S & Passatore M (2008). Autonomic effects on skeletal muscle. In Encyclopedia of Neuroscience, ed. Binder MD, Hirokawa N & Windhorst U, pp. 250–253. Springer, Berlin, Heidelberg New York (DOI: 10.1007/978-3-540-29678-2).