
Physiology News Magazine
Neuronal lactate metabolism
The first piece of the jigsaw falls into place
Features
Neuronal lactate metabolism
The first piece of the jigsaw falls into place
Features
Angus M Brown
MRC Applied Neuroscience Group, School of Biomedical Sciences
Malcolm J W Prior
The Brain and Body Centre, School of Physics and Astronomy
University of Nottingham, Nottingham, UK
https://doi.org/10.36866/pn.64.23

In a recent article we described the potential of systemic lactate to act as an energy substrate for the brain under specialised conditions (Brown & Prior, 2004), and suggested that MRI could be used to answer key questions regarding this issue. We have now carried out some preliminary experiments and present the essential findings here. However, it is perhaps expedient to backtrack and provide some background on brain energy metabolism in order to present our data in the appropriate context.
That glucose is the main energy support of the brain is in no doubt, as previously described (Brown & Prior, 2004). Key facts of brain energy metabolism are as follows:
(1) although it comprises only 2% of body weight, the brain takes up 20% of available oxygen and 40% of available glucose via 10% of the cardiac output, thus contributing to 20% of the basal metabolic rate, i.e. resting brain has a very high metabolic demand.
(2) the respiratory quotient (RQ) of the brain, the ratio of the CO2 produced to O2 consumed, is close to 1.0, implying that efficient oxidative phosphorylation of carbohydrate (glucose) is the predominant energy generating process in the brain.
(3) the metabolic ratio of the brain (the cerebral metabolic rate of oxygen consumption (CMRO2) versus cerebral glucose utilisation (CMRglc) at rest is close to 6 (~5.7), which signifies almost complete oxidation of glucose.
Glucose (C6H12O6) + 6 O2 → 6 CO2 + 6 H2O
The requirement of integrated brain function for oxygen has been amply (if not altogether ethically) demonstrated (Rossen et al. 1943). During the Second World War, Anderson’s group persuaded the authorities in the USA to allow them to carry out experiments on some 140 prisoners in the Stillwater State Prison, Minnesota. They implemented a Mae West type lifebelt, which could inflate a pneumatic cuff to occlude the carotid arteries, while leaving the trachea unobstructed (Fig. 1). They found that in all ‘volunteers’ consciousness was lost 6 to 8 seconds after occlusion of the carotid arteries, thus demonstrating the absolute dependence of higher cognitive function on a continuous delivery of oxygenated blood to the brain. Thus the conventional view of brain energy metabolism can be summarised as follows: the brain requires a constant uninterrupted supply of both blood borne glucose to fuel brain energy metabolism, and oxygen as a means to efficiently metabolise the glucose.
In recent years there have been two main challenges to this dogmatic view. The first concerns the coupling of glucose utilisation and oxygen uptake in the brain during activation. It was shown by Raichle’s group that during increased brain activation the metabolic ratio decreases, implying that glucose utilisation is not matched by increased oxygen uptake. This leads to the conclusion that there is a significant increase in glycolytic metabolism in activated brain areas (Fox et al. 1988). Given the dependence of brain function on an adequate supply of oxygen it may appear puzzling that a small amount of glucose is not oxidised completely at rest (metabolic ratio ~ 5.7). This may be due to:
(1) biosynthesis – glutamate and GABA, the two most common neurotransmitters in the brain, are formed via the Krebs cycle intermediary α-ketoglutarate, but are ultimately derived from glucose.
(2) at rest glycolysis exceeds the oxidative capacity of the brain, thus lactate produced in excess of demand is lost from the brain.
(3) there is a lack of mitochondria at synapses, dictating glycolytic metabolism at these sites.
Additionally, not all brain tissue requires oxidative phosphorylation. Astrocytes are widely believed to be glycolytic (see later), but some central tissue such as the optic nerve axons can survive in the absence of oxygen (Baltan Tekkök et al. 2003). However, during activation of brain areas the uncoupling of glucose and oxygen is too great to be due to the above explanations, and it remains an intriguing mystery.
The second challenge to the dogmatic view of brain energy metabolism relates to the compartmentalisation of glucose once it has crossed from the circulation into the brain parenchyma. It has been argued that astrocytes may take up glucose and glycolytically metabolise it to lactate, before passing the lactate the neurones. This is the essence of the ‘astrocyte neurone lactate shuttle’ (ANLS) hypothesis (Pellerin & Magistretti, 1994; 2003). They argue that the system is activated during synaptic activity where astrocytic reuptake of synaptic glutamate (coupled with Na+ uptake) promotes activation of the Na+ pump and conversion of glutamate to glutamine. Both these processes require ATP, which could be provided by glycolytic metabolism of glucose. The end product of this astrocytic glycolysis is lactate, which is subsequently transported to neurones for oxidative metabolism (Fig. 2). Such a system does have an appeal, namely:
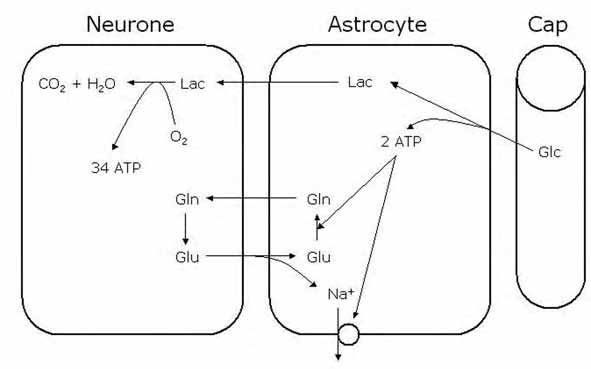
(1) it correlates well with the coupling of glucose consumption to glutamate cycling.
(2) increasing glutamate levels leads to increased glucose uptake by astrocytes.
(3) it explains the disproportionate uptake of glucose into glial cells (50%) relative to their metabolic rate (5%).
(4) lactate is released from astrocytes.
The ANLS is hypothesised to be activated during increased brain activity. Thus basal brain activity is adequately served by glucose metabolism, but increases in tissue demand require that lactate is metabolised by neurones. This ANLS hypothesis has caused a major spar in the brain energy metabolism community, and has essentially polarised the field, with the interested parties taking pot shots at each other at monthly intervals via reviews published in the Journal of Cerebral Blood Flow and Metabolism. It would seem logical, if this issue is so contentious, to design a simple experiment(s) to directly measure cellular substrate metabolism. It is here that the divergence between desire and reality has led to great confusion. This can be summarised as follows:
Desire – to measure energy metabolism in a single cell (or even different regions of a single cell) in the brain in real time.
Reality – the heterogeneous nature of the cellular organisation of the brain, coupled with the limited spatial and temporal resolution of currently available monitoring techniques, makes this impossible.
Compromise – devise experiments using currently available techniques whose results can be used to infer unknown aspects of metabolism that cannot currently be measured directly.
It is this compromise that has led to confusion, as groups have clashed over interpretation of data. However, a key issue raised by both challenges to the dogmatic view of brain energy metabolism is neuronal metabolism of lactate. That brain tissue can use lactate as an energy substrate has been known for over 50 years, although these experiments were carried out on in vitro brain preparations (McIlwain, 1953). More recent studies have shown that, although lactate can support energy levels in brain slices, the electrophysiological indices of function (the EPSP and population spike) are not fully supported, implying an absolute requirement for glucose that lactate cannot provide (Bachelard et al. 1984). This has recently been substantiated by studies showing that synaptic mechanisms require glucose, and that lactate cannot act as a substitute (Bak et al. 2006). This may be due to the positioning of glycolytic enzymes at the cell surface adjacent to Na+ pumps, or due to the lack of mitochondria at synapses. That lactate can at least partially support function implies that all the necessary transporters and enzymes are in place for efficient lactate metabolism by neurones.
Indeed, studies on rodent optic nerve have demonstrated that glycogenderived astrocytic lactate is shuttled to fuel axons both during aglycaemia and during increased tissue energy demand under normoglycaemic conditions (Brown et al. 2003).
An interesting and seemingly unrelated series of studies have revealed that, under certain conditions, it appears that lactate in the systemic circulation can act to fuel the brain. During extreme exercise blood glucose levels fall while blood lactate levels can increase dramatically, resulting in a net uptake of lactate into the brain (Dalsgaard et al. 2004). However, a net uptake of lactate, although suggestive of lactate metabolism, does not definitively show that lactate is metabolised by the brain. Sonnewald’s group has addressed this issue where labelled lactate (13C) was systemically injected in control animals under normoglycaemic conditions resulting in an increased labelled alanine in the brain (Qu et al. 2000). We have taken this idea a step further and have injected labelled lactate into rats that were rendered hypoglycaemic by injection of insulin. We hypothesised that under these conditions there should be minimal gluconeogenesis of lactate in the liver as there is no excess systemic glucose for storage. Transient blood lactate levels of 10 mM were reached while glucose levels fell to about 2 mM. NMR spectroscopy was used to determine enrichment of metabolite 13C from brain extracts. We detected labelled lactate and glutamate, but a complete lack of labelled glucose (Fig. 3), indicating that no glucose from gluconeogenesis was present in the brain tissue. This implies that the labelled glutamate, an indicator of oxidative metabolism, must have originated from lactate.
The uptake and metabolism of systemic lactate by the brain can be seen as an extension of the cell-to-cell signalling concept to include peripheral tissue. The data from studies on vigorous exercise have revealed the ability of muscle cells to convert glucose to lactate, which is then extracted by the brain from the systemic circulation and metabolised. This highlights the ability of tissues to partially metabolise glucose, then pass on the energy rich intermediary metabolites to different cells or even tissues. This ensures an efficient use of glucose, a concept that has been summarised as ‘activitydependent and temporal-spatial partitioning of brain metabolism’ (Dienel, 2004).
As we go to press, another J Cereb Blood Flow Metab review by Shulman’s group (Hyder et al. 2006), leading advocates of the ANLS hypothesis, proposes an updated model of glial-neuronal metabolic trafficking to incorporate oxidative glucose metabolism in astrocytes as well as significant neuronal uptake of glucose. The plot thickens!
References
Bachelard HS, Cox DW & Drower J (1984). Sensitivity of guinea-pig hippocampal granule cell field potentials to hexoses in vitro: an effect on cell excitability? J Physiol 352, 91-102.
Bak LK, Schousbo A, Sonnewald U & Waagepetersen HS (2006). Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab (in press).
Baltan Tekkök S, Brown AM & Ransom BR (2003). Axon function persists during anoxia in mammalian white matter. J Cereb Blood Flow Metab 23, 1340-1348.
Brown AM, Baltan Tekkök S & Ransom BR (2003). Glycogen regulation and functional role in mouse white matter. J Physiol 549, 501-512.
Brown AM & Prior MJW (2004). Conditions under which systemic lactate may act as a metabolic substrate for the brain. Physiology News 56, 20-21.
Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T & Seche NH (2004). A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol 554, 571-578.
Dienel GA (2004). Lactate muscles its way into consciousness: fueling brain activation. Am J Physiol Regul Integr Comp Physiol 287, R519-521.
Fox PT, Raichle ME, Mintun MA & Dence C (1988). Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462-464.
Hyder F, Patel A, Gjedde A, Rothman D, Behar K & Shulman R (2006). Neuronal-glial glucose oxidation and glytamatergic-GABAergic function. J Cereb Blood Flow Metab 26, 865-877.
McIlwain H (1953). Substances which support respiration and metabolic response to electrical impulses in human cerebral tissues. J Neurol, Neurosurg Psychiatry 16, 257-266.
Pellerin L & Magistretti PJ (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91, 10625-10629.
Pellerin L & Magistretti PJ (2003). Food for thought: challenging the dogmas. J Cereb Blood Flow Metab 23, 1282-1286.
Qu H, Haberg A, Haraldseth O, Unsgard G & Sonnewald U (2000). 13C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci 22, 429-436.
Rossen R, Kabat H & Anderson JP (1943). Acute arrest of cerebral circulation in man. Arch Neurol Psychiatry 50, 510-528.
