
Physiology News Magazine
Not for giant axons only
Pictures in the skin. Insights into fundamental functions from familiarity with the whole by Andrew Packard
Features
Not for giant axons only
Pictures in the skin. Insights into fundamental functions from familiarity with the whole by Andrew Packard
Features
Andrew Packard
Stazione Zoologica ‘Anton Dohrn’, Naples, Italy andrew@packards.de
https://doi.org/10.36866/pn.52.16

It is not just for ethical and legal reasons that most laboratories have abandoned the whole animal approach to physiology. So perhaps I have been unusually lucky that my squids have still to be closed off on the far side of the animal house door in the name of tidiness and division of labour. That I am able to practise the art that served our predecessors for hundreds of years: feeding, caring, and generally getting to know each experimental individual: part of a procedure I have called ontophysiology. For with cephalopods, where colour is concerned (Packard, 1995 for summary), one can follow activity simultaneously at the behavioural and at intermediate levels down to the cellular. It is simply a matter of appropriate magnification – and of attitude.
The field is wide open and the intellectual rewards of letting the animal tell its own story are great.
Access to brain functions through the systematics of colour: the whole informs the parts
Edmund V.Cowdry (1888-1975), is better known for his contributions to medicine than for constructing an ‘octopus car’ when an anatomy student at the University of Toronto. His faithful water-colours of octopuses placed in this device in front of the marine biological station of Bermuda (Figure 1a,b) are amongst the first attempts to classify the patterns of cephalopod molluscs (Cowdry 1911). Commuting between Plymouth, Oxford, London and Naples in search of cuttlefish and octopuses, J.Z.Young, William Holmes and Brian Boycott added to the catalogue over the years. (Young’s drawing of the male of a local species displaying to a female, spotted while snorkelling between sessions of external examining in Singapore, is one of the few records of octopus courtship). So when Geoff Sanders,Young’s psychology assistant in Naples, walked into my office with an armful of notes on the various postures and patterns observed during training experiments, he was unknowingly continuing the tradition of noting down – and sketching – any new natural behaviours seen during the course of experiments on quite different things.


Our job was to fill the gaps in the ethological description and classification, and to parse the patterns. Boycott had obtained centrally induced expressions of colour by electrical stimulation of motor centres in the brain, and before him Enrico Sereni (Young’s mentor when he was Oxford scholar at Naples) had tried pharmacological stimulation: a route later adopted by J.B.Messenger and colleagues. It was a short step to discover that the irreducible (parsed) components of the patterns corresponded to hardwired units in brain and skin which could be topically stimulated with an exploring electrode.
At which point I naturally turned to the isolated skin, only to find – lesson number one – that the isolated preparation behaves very differently from, and much less reliably than, the animal in its entirety.
Unilateral denervation…
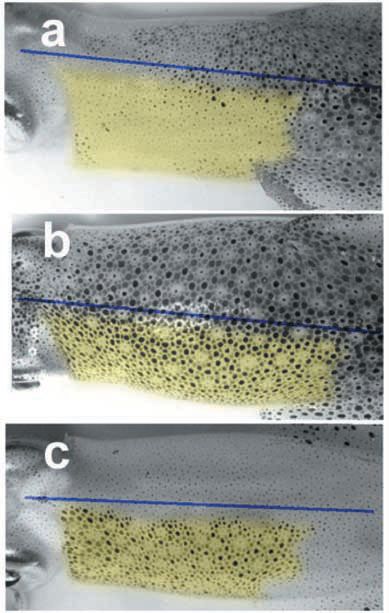
Although something of the wonder is now lost to us, the Belgian physiologist Léon Fredericq (1851–1935) – who also was a talented water-colourist (!) – discovered that when he reached through the breathing aperture of an octopus to cut the pallial nerve on one side, half of the mantle immediately went pale. The same approach was used by Sanders and Young (1978) to very good effect in a rarely quoted study of pattern regeneration.
The paling of the skin following the initial cut does not last, however. At two days, as the mixed pallial nerve degenerates and denervation supersensitivity develops in the chromatophore muscles, the operated side gradually becomes dark brown (compare Fig. 2).

… and the myogenic signs that result
Chromatophores (spots) are much larger in squids than in octopuses. The skin receives oxygen directly through a surface of exceptionally good optical quality, so that individual and collective signals generated by muscular tension on the spots can be recorded at relatively high resolution (Fig. 3) and for many hours (even days) following brain death. The tonic darkening of denervation supersensitivity (DDSS) is best seen when the rest of the animal is pale (Fig. 2 & 4c). Each spot in these configurations has a round profile, roundness resulting from equal and synchronous contraction of most (or all) of the 20 or so radial muscle fibres supplying any given chromatophore. These muscle fibres are coupled and their syntony and synchrony are myogenic.
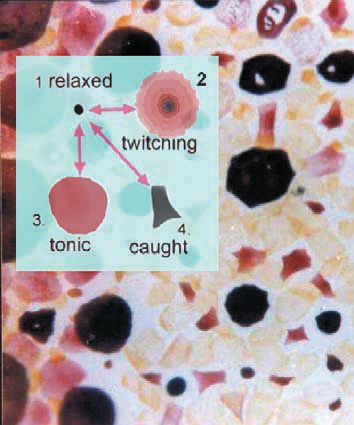
Nevertheless, the configurations are remarkably similar to patterns also seen on the intact side (Fig. 4b), as if the nerve had never been cut – or as if those normal patterns were also myogenic!
Subsequently (four to eight days depending on temperature) DDSS gives way to coherent but irregular waves of twitch contractions propagating at ~1 cm/s throughout the denervated territory – sign that nerve degeneration is complete. Like the tonic configurations, which can be regarded as slow or standing waves of contractile state, these fast waves exhibit chaotic dynamics. (*1)
Development the key to understanding coupled ensembles
Fortunately, there are some simple rules for reducing the complexity of the picture at this stage, when everything seems to be communicating with everything else. In cephalopods, as if their other gifts to the experimenter were not enough, the ontogenetic history of each chromatophore organ is encoded in its position, in its size and in its colour (largest-darkest oldest, smallest-lightest youngest) (Fig.5).
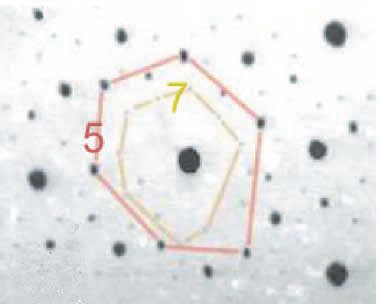
With this knowledge, it takes only a few seconds of the hundreds of hours of videorecordings of myogenic automaticity to realize that waves tend to run selectively through yellow, orange, red, and brown spots (of several sizes) lying in closely superimposed networks. Within any one network, connectivity (measured as probability of transmission of contractile state) is normally 100%. Between colour classes it is differential. (*2)
I have given the name homeotaxy to the observed conformity of behaviour among elements of a given developmental class when freed from nervous control. It persists for as long as the skin remains alive. Is homeotaxy (Greek: same arrangement, or peer conformity to give it an English name) a characteristic of waves propagating through other coupled ensembles?
Complementary roles of spots in pattern generation…
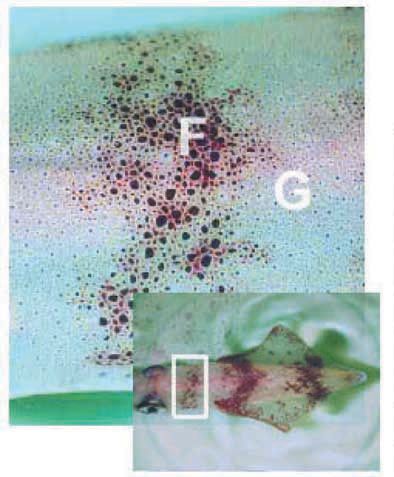
Recently, squids have helped resolve a problem that had plagued me for decades. One class of pattern – in Nature as in Art – consists of both figure and ground. The tattered bar that appears across the front of the mantle when a squid is hiding near the bottom of the tank (Fig. 6) is one such feature (F). It behaves more or less like classical motor units being generated by twitches and tetani that expand certain dark spots. Increases in frequency of firing increase both the sizes of individual spots and the numbers of spots responding: a process of spatial recruitment. Many of these spots have polygonal profiles in close up view (Fig. 6): the shape telling which of the 20-25 muscle fibres surrounding each spot are developing tension. However, the many featureless uniform and graded colours in the repertoire of squids, which also furnish the ground (G) to these bars, are not organised in clearcut motor units. They are built up of round spots – like the myogenically expanded spots encountered in denervated skin.
Opponent processing for the perception of contrast – necessary also for the discrimination of figure and ground – occurs early on in the vertebrate visual pathway, classically through lateral inhibition. Contrast is generated in the skin of octopuses, by what appears to be lateral inhibition, in situations of behavioural conflict and as a major component of camouflage patterns (Packard, 1995). There has long been an argument whether chromatophore muscle fibres receive inhibitory innervation, though none has ever been found. After carefully comparing the colour of a pale octopus on a sandy ground and when ‘frightened’ (a pattern now known as dymantic, or deimatic) the young Cowdry concluded that there was some form of inhibition: though not necessarily nervous (see Box p.16).

How active, rather than passive, relaxation of chromatophores is achieved is still not clear. But the whiteness of contrast – and of the ‘frightened’ octopus – can be simulated by subcutaneously injected serotonin and by the nitric oxide donor DEANO. (*3) The reaction propagates as a slow wave of relaxation and is stronger in denervated than in innervated skin.
… mediated by reciprocal inhibition between muscle fibres?
Generation of features (F) against a clear ground (G) through opponent processing need not in principle involve nerves. One piece of evidence for its occurring as reciprocal inhibition between different muscle fibres is shown in Figure 7 presented at last year’s LiverpooI meeting (Packard, 2002). I have since found that the phenomenon, regularly seen post mortem when the nerve has died or become anoxic, also occurs in the operated half of a long-term unilaterally denervated mantle, i.e. in the total absence of innervation.
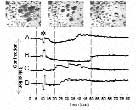
If reciprocity is occurring between two muscle fibre types – either directly or indirectly through an interstitial cell layer – this would be a new finding for muscle generally. It would make sense. Most visceral and other kinds of muscle in the animal kingdom is in blocks that require relaxation of muscle in some directions while those in the orthogonal are contracting. Fundamental properties such as this have not evolved just for generating pretty pictures in the skin.
References
Cowdry EV (1911). The colour changes of Octopus vulgaris Lmk. Contributions from the Bermuda Biological Station for Research No. 22 and from the University of Toronto Studies. Biological Series, no. 10.
Packard A (1995). Organization of cephalopod chromatophore systems: a neuromuscular image-generator. In Cephalopod Neurobiology, eds. Abbott NJ, Williamson R & Maddock L, pp.331368. Oxford University Press, Oxford.
Packard A (2002) Myo-muscular interactions: evidence for reciprocal inhibition between classes of muscle fibre. J. Physiol 543P, 104P.
Sanders GD & Young JZ (1978). Reappearance of specific colour patterns after nerve regeneration in Octopus. Proc Roy Soc Lond 186, 1-11.
*Notes
1: An exhaustive description of these waves requires video clips and has never been published. Some may be consulted on http://www.gfai.de/www_open/perspg/g_heinz/biomodel/squids/squid s.htm others on CD from the author.
2: Fast waves can be reversibly interrupted by topically applied heptanol (which is both anaesthetic and gap junction blocker). RF frequencies from a mobile ‘phone interfere with their generation. For an eye-opening introduction to mechanism(s) that might be involved in these (usually hidden) kinds of horizontal conduction, I recommend the review of Ho and Knight (Ho M-W & Knight DP (1998). The acupuncture system and the liquid crystalline collagen fibres of the connective tissues Am J Chin Med 26(3-4), 251- 263.
3: With thanks to Anna Palumbo.
