
Physiology News Magazine
Nucleotide release and airway epithelial physiology
Nucleotide release provides extracellular communication in poorly innervated tissues. In airway epithelia, synchronous release of nucleotides and mucins ensures efficient lung innate defence
Features
Nucleotide release and airway epithelial physiology
Nucleotide release provides extracellular communication in poorly innervated tissues. In airway epithelia, synchronous release of nucleotides and mucins ensures efficient lung innate defence
Features
Silvia M Kreda, Richard C Boucher, & Eduardo R Lazarowski
Cystic Fibrosis/Pulmonary Research and Treatment Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
https://doi.org/10.36866/pn.71.19

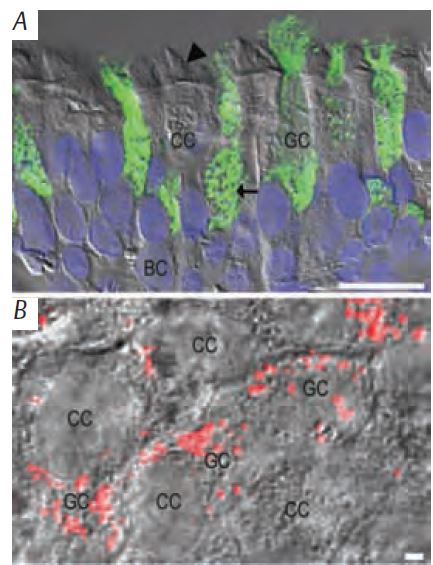
The airway epithelium is a complex tissue comprising at least three distinct cell types (ciliated, goblet, and basal cells; Fig. 1A). How does this complex epithelium, which is poorly innervated and exhibits little intercellular coupling, coordinate its activities? The answer is: nucleotide release.
Nucleotides, of which adenine-triphosphate (ATP) is the best known, not only are part of our nucleic acids, phosphorylation and reducing reactions, and signal transduction molecules, but also act as pharmacological ligands for purinergic receptors. Remarkably, extracellular ATP has been detected in the majority of non-excitatory tissues, including most epithelia, endothelia, smooth muscle, fibroblasts, astrocytes and blood cells. The significance of nucleotides as extracellular signalling molecules is emphasised by the ubiquitous distribution of several families of ectoenzymes that catalyze nucleotide breakdown and inter-conversion. Thus, not just ATP, but also its products of ectoenzyme activity (e.g. ADP, AMP and adenosine), are present in the extracellular milieu. Together, they elicit a varied array of physiological responses downstream from at least 19 different nucleotide-activated (purinergic) cell surface receptors (Lazarowski et al. 2003). In the lung, nucleotides and their metabolites are present in the airway surface liquid (ASL), a thin layer composed mainly of water, electrolytes and gel-forming mucins, that bathes the epithelial surface. ASL nucleotides regulate lung innate defence activities. Purinergic receptors on ciliated cells regulate ciliary beating and ASL volume via ion and water transport, and on goblet cells, purinoreceptors modulate mucin secretion. These activities are finely tuned to allow effective mucociliary clearance, an important element of the lung innate defence.
The balance between liquid secretion (ion and water transports), mucin secretion (granule exocytosis), and ASL transport (ciliary beating) ensures the rapid removal of inhaled foreign materials. The ‘imbalance’ of these epithelial defence activities leads to severe lung disease. COPD, cystic fibrosis and primary ciliary dyskinesia are examples of obstructive lung disease originated by lumenal mucus accumulation, ASL volume depletion, and ineffective cilia beating, respectively (Knowles & Boucher, 2002).
Purinergic regulation of native defence activities occurs chiefly on the lumenal surface of epithelial cells. Indeed, the activity of releasing nucleotides into the ASL resides within the epithelial cells themselves. Only in the last 10 years has it been understood that cells can regulate the release of ATP and other nucleotides (i.e. nucleotide-sugars and uridine nucleotides) by mechanisms that do not involve cell-lysis (Lazarowski et al. 2003; Kreda et al. 2007). In the airways, ATP is released in response to a variety of stimuli, and likely reflects the histological complexity of this epithelium. Thus, there is no consensus about the mechanism(s) of nucleotide release, and a combination of both conductive and exocytotic pathways may be responsible for epithelial nucleotide secretion. A conductive mechanism would involve a cell surface channel or transporter that release nucleotides directly from the cytoplasm. An exocytotic mechanism would require the trafficking of vesicles to the cell surface. These vesicles could contain nucleotides and/or conductive molecules to be released into the extracellular milieu or inserted into the plasma membrane, respectively. However, with the exception of purinergic neurons and a few non-excitatory cell types (e.g. platelets), nucleotide-containing granules are not apparent in most non-excitatory cell types, including epithelial cells.
Recent work by our and other groups (Kreda et al. 2007; Tatur et al. 2007), suggests that nucleotide release from airway epithelial cells does encompass exocytosis of nucleotide-containing vesicles. Airway epithelial goblet cells express gel-forming mucin secretory granules (Fig. 1B and 2A), which are released onto the lumenal surface by Ca2+-dependent exocytosis. Goblet cells and neurons employ a similar exocytotic machinery to secrete mucin granules and synaptic vesicles, respectively. The fact that ATP and uridine-diphosphate-glucose (UDP-glc) are concentrated within the endo-plasmic reticulum and Golgi, where they facilitate glycosylation, phosphorylation and folding of secretory proteins, suggests that nucleotides could be stored within mucin secretory granules during granule assembling and ‘hitch hike’ with mucins for the ‘exocytotic ride’ to the airway lumen. Notably, sputum specimens from cystic fibrosis individuals exhibiting airway mucin hypersecretion contain remarkably high levels of nucleotides relative to healthy subjects (Lazarowski et al. unpublished).
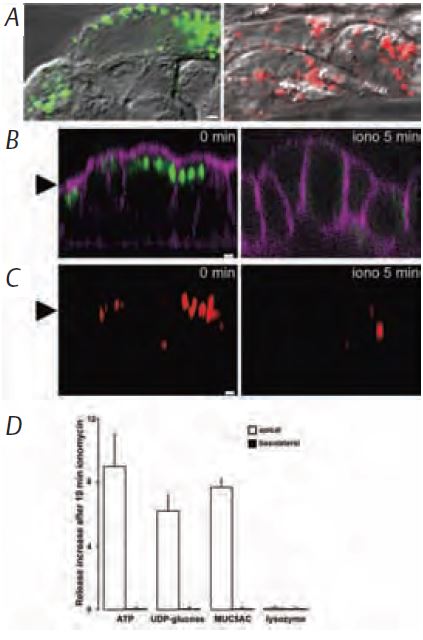
Using biochemical and confocal microscopy approaches, we recently determined that ATP and UDP-glc are released lumenally from goblet cell lines by receptor-activated, Ca2+-dependent exocytosis (Fig. 2; Kreda et al. 2007). The kinetics of ATP release and mucin-granule secretion were similar and triggered by identical stimuli. Moreover, quinacrine, which labels ATP-rich granules in secretory cells, accumulated in the granules of airway goblet cells. These quinacrine granules were released concomitantly with ATP and gel-forming mucin granules into the lumenal surface by Ca2+-dependent exocytosis (Kreda et al. 2007).
In summary, our latest data suggest that a pool of nucleotides is released simultaneously with mucins by Ca2+-dependent exocytosis. Although the possibility that nucleotides are concentrated within mucin granules remains to be confirmed, the finding that nucleotides and mucins are being ‘coordinately’ secreted is relevant to ASL regulation. Mucins are condensed in granules and upon secretion they expand greatly as a gel and disperse into the ASL. Ion and water transport activities are, therefore, necessary for mucin hydration and dispersion; however, these activities are absent from goblet cells. We hypothesise that ATP is coordinately released with mucins and, therefore, it can signal in a paracrine fashion ciliated cells to increase ion and water transport (and cilia beating) to hydrate and disperse secreting mucins in the ASL (Fig. 3). Regulated nucleotide release is the key to coordination of these epithelial activities. Good coordination, good breathing, good health.

Acknowledgements
The authors gratefully thank Catja van Heusden for HPLC expert technical assistance and Lisa Brown for the editing of the manuscript. This study was supported by grants from the Mary Lynn Richardson Fund, USA (to SMK), NIH P01-HL34322 (to SMK and ERL).
References
Knowles MR & Boucher RC (2002). Mucus clearance as a primary innate defense mechanism for mammalian airways J Clin Invest 109, 571–577.
Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR & Boucher RC (2005). Characterization of wild-type and {Delta}F508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16, 2154–2167.
Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC & Lazarowski ER (2007). Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584, 245–259.
Lazarowski ER, Shea DA, Boucher RC & Harden TK (2003). Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63, 1190–1197.
Tatur S, Groulx N, Orlov SN & Grygorczyk R (2007). Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J Physiol 584, 419–435.
