
Physiology News Magazine
Olfactory marker protein: a gift to molecular biologists, an enigma to physiologists
Olfactory marker protein (OMP), a small cytoplasmic protein almost exclusively expressed in nasal chemosensory cells, has made major contributions to research progress in olfaction ever since its discovery over 35 years ago. However, the function of OMP and its mechanism of action has remained an enigma. Slowly, answers to these questions are beginning to emerge
Features
Olfactory marker protein: a gift to molecular biologists, an enigma to physiologists
Olfactory marker protein (OMP), a small cytoplasmic protein almost exclusively expressed in nasal chemosensory cells, has made major contributions to research progress in olfaction ever since its discovery over 35 years ago. However, the function of OMP and its mechanism of action has remained an enigma. Slowly, answers to these questions are beginning to emerge
Features
Johannes Reisert (1) & Frank L Margolis (2)
1: Monell Chemical Senses Center, Philadelphia, PA, USA
2: Department of Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore MD, USA
https://doi.org/10.36866/pn.73.27

Everyone working in the field of chemosensation knows OMP, but nobody knows its function. A PubMed search for ‘olfactory marker protein’ yields around 350 publications, but when probing deeper to identify what OMP does physiologically at the cellular or molecular level, this number drops to single digits. Antibodies to OMP have been used to characterize olfactory receptor neurons (ORNs) in numerous species and the OMP gene has been used to generate transgenic mice with ORN-specific expression profiles in hundreds of publications. However, to date only one protein has been identified that interacts with OMP, and that might provide insight to help elucidate OMP’s function. So why is OMP so widely utilized, while so little is known about its function?

OMP is a small (~160 amino acids) cytoplasmic protein (Margolis, 1972). Its amino acid sequence is >50 % identical in all vertebrate species but has no known sequence motifs. OMP is abundantly and virtually exclusively expressed in mature chemosensory neurons in the nasal cavity (Fig. 1). As such OMP has been a valued and cleverly exploited tool to identify and label vomeronasal and ORNs, neurons of the septal organ and also contributed to the recent rediscovery of the Grueneberg ganglion, a cluster of cells located in the anterior nasal cavity. Furthermore, the OMP promoter or the OMP locus has been used extensively to drive expression of desired genes in chemosensory neurons. Examples are the markers thy1.1, lac-Z or GFP, which have helped to describe fundamental connectivity patterns of olfactory receptor neuron axons to their glomerular targets in the olfactory bulb (e.g. Danciger et al. 1989; Mombaerts et al. 1996), direct expression of a single identified olfactory receptor to every ORN (instead of a randomly chosen one), or expression of the fluorescent exocytosis indicator synaptopHluorin to image bulbar activity patterns (Bozza et al. 2004).
Not until the generation of an OMP knockout (Buiakova et al. 1996) was it actually certain that OMP has a role in olfactory transduction (see Table 1 for biological functions of OMP). Immunohistochemical and anatomical analyses of OMP-/-olfactory epithelium revealed no differences in morphology or protein expression, suggesting that OMP may not play a developmental role.
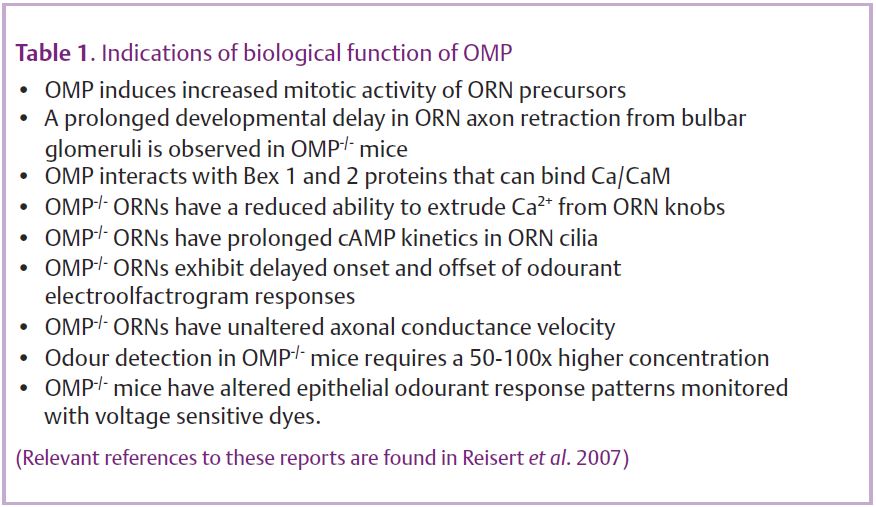
Interestingly, OMP-/- ORN axons are compromised in their ability to properly target the correct glomerulus in the bulb (St John & Key, 2005). In addition, the olfactory bulbs of OMP-/- mice are smaller and display reduced tyrosine hydroxylase activity and CCK content, changes also observed in bulbs of odour-deprived wild-type mice. These observations imply that OMP alters afferent input to the bulb by modulating the activity of ORNs, which is indeed the case. The odour-induced electrolfactogram, a mass epithelial recording, revealed that ORNs which lack OMP have a response, where not a single, but at least three aspects are slowed: response delay, time to peak and the response termination (Buiakova et al. 1996). Single cell recordings confirmed these findings and also revealed that odour-induced action potential patterns were changed (Fig. 2). Action potentials were generated with a 3-fold longer delay after stimulation onset and firing persisted for longer compared to wild-type recordings (Reisert et al. 2007), both possibly contributing to the bulbar changes observed in OMP knockouts. OMP-/- ORNs also showed a severely reduced ability to recover from adaptation due to the 10-fold slowed termination of the receptor current, an effect which might also contribute to the observed 50-fold reduction in odour sensitivity of OMP-/- mice when tested behaviourally (Youngentob & Margolis, 1999).
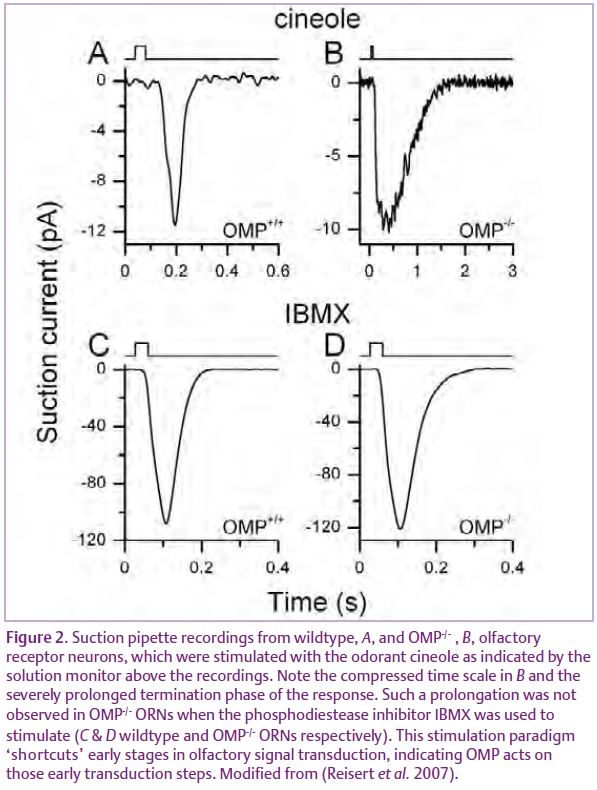
These dramatic and multiple effects of OMP on the odour response suggest that it must function at a regulatory focal point in ciliary olfactory transduction. In ORNs activation of an odorant receptor leads, via a G protein, to adenylyl cyclase activation, increase in ciliary cAMP and activation of a Ca2+ permeable cyclic nucleotide-gated (CNG) channel, followed by opening of an excitatory Ca2+-activated Cl-channel. Response termination occurs by degradation of cAMP by a CaM-dependent phosphodiesterase and removal of Ca2+ by a Na+/Ca2+ exchanger and PMCA activity to close the CNG and the Cl- channel respectively. On which or on how many transduction components might OMP exert its effect? cAMP kinetics have been found to be greatly prolonged in OMP-/- ORNs suggesting that either cAMP production itself is prolonged or degradation slowed. The former is the case, suggesting that the action of OMP in the cilia may be restricted to the early stages of signal transduction prior to cAMP production: the odorant receptor, G protein and/or the adenylyl cyclase (Reisert et al. 2007). Hence OMP’s role is to speed up the odorant response, which might contribute to fast sampling of odour cues and allowing ORNs to re-sensitize quickly. This is consistent with the alterations in odorant-induced mucosal activity patterns in OMP-/-mice (Youngentob et al. 2003).
Nevertheless, many questions remain. Does OMP act directly on the previously mentioned transduction components? Probably not, since vomeronasal neurons also express OMP but use an entirely different PLC based-transduction pathway. Similarly OMP positive cells of the Grueneberg ganglion express, besides odorant receptors, trace amine-associated and vomeronasal receptors. This implies the presence of a protein common to all OMP-expressing cells through which OMP exerts its function on a range of quite different transduction components. A possible candidate might be members of the family of brain expressed X-linked (Bex) genes which have been found to interact with OMP and to be present in ORNs of the main and vomeronasal epithelium (Koo et al. 2005).
On the other hand, if OMP is truly a more general modulator of signal transduction cascades, why is its expression so highly restricted to nasal chemosensory neurons, but not to other (e.g. chemosensory taste or gut) cells? The absence of OMP orthologs in invertebrates suggests that it is not required per se for chemosensory detection. Furthermore it can also not be assumed that OMP only has a role in chemosensory signal transduction, since it does not localize exclusively to the cilia or vomeronasal microvilli, but is present throughout the entire cell.
Hopefully, in the coming years, OMP will become less of an enigma and more of a gift to physiologists too by revealing its function and providing new insights into signal transduction mechanisms.
References
Bozza T, McGann JP, Mombaerts P & Wachowiak M (2004). In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron 42, 9–21.
Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL & Margolis FL (1996). Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci USA 93,
9858–9863.
Danciger E, Mettling C, Vidal M, Morris R & Margolis F (1989). Olfactory marker protein gene: its structure and olfactory neuron-specific expression in transgenic mice. Proc Natl Acad Sci USA 86, 8565–8569.
Koo JH, Saraswati M & Margolis FL (2005). Immunolocalization of Bex protein in the mouse brain and olfactory system. J Comp Neurol 487, 1–14.
Margolis FL (1972). A brain protein unique to the olfactory bulb. Proc Natl Acad Sci USA 69, 1221–1224.
Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J & Axel R (1996). Visualizing an olfactory sensory map. Cell 87, 675–686.
Reisert J, Yau KW & Margolis FL (2007). Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol 585, 731–740.
St John JA & Key B (2005). Olfactory marker protein modulates primary olfactory axon overshooting in the olfactory bulb. J Comp Neurol 488, 61–69.
Youngentob SL, Kent PF & Margolis FL (2003). OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol 13, 13.
Youngentob SL & Margolis FL (1999). OMP gene deletion causes an elevation in behavioral threshold sensitivity. Neuroreport 10, 15–19.
