
Physiology News Magazine
Organization of intermediary metabolism – lactate and its transporters
Features
Organization of intermediary metabolism – lactate and its transporters
Features
George A Brooks & Takeshi Hashimoto
Integrative Biology, University of California, Berkeley, CA, USA
https://doi.org/10.36866/pn.65.19

Once thought to be a ‘dead-end’ metabolite produced because of O2 insufficiency, lactate is produced continuously under fully aerobic conditions in humans and other mammals. This production serves several functions, key of which is that it is permissive of glycolysis. Lactate is removed mainly by oxidation in red skeletal and cardiac muscle fibres, but also by the liver and kidneys where lactate is the major gluconeogenic precursor. By affecting redox in cells as well as in cell compartments of removal, lactate serves as a signalling molecule. Lactate traverses cell membranes by means of several monocarboxylate (lactate/pyruvate) transport proteins (MCT). Our recent work using microscopy and a variety of techniques shows that MCTs and related proteins occupy cell domains to facilitate the exchange and use of lactate.
Lactate is an end product of glycogenolysis and glycolysis produced only when cells lack oxygen: ‘this I learned during my undergraduate studies in Japan’, said Takeshi Hashimoto. ‘Well, that’s what I learned also as an undergraduate in the 1960s’, replied George Brooks. ‘But’, Brooks went on to add, ‘as a graduate student and ever since, I could not find proof that lactate is the consequence of O2limited metabolism in humans or other mammals.’ Rather, the O2 debt ideas are traceable to early 20th century studies on non-circulated and nonoxygenated amphibian muscle preparations. But now, based on the recent report of Hashimoto et al. (2005), as well as related prior investigations, it is time to turn over the classical idea of lactate production due to O2 lack. Today, we can think of lactate as a substrate for mitochondrial respiration that can be shared among cell compartments, tissues and organs. As well, lactate serves as a gluconeogenic precursor, and as a signalling molecule, a ‘lactormone’ because of its influence on redox. And, perhaps physiologically more significant is that lessons learned on muscle can be generalized to the functioning of other cells and tissues, including brain (Brooks, 1998).
Based on isotope tracer, arterial-venous difference mass balance and biopsy studies (Brooks et al. 1991) we now know that skeletal muscle is not only the major site of lactate production, but also the major site of its removal, mainly via oxidation. Lactate and pyruvate are exchanged across muscle cell (sarcolemmal) membranes by facilitated, proton-linked transport (Roth & Brooks, 1990) involving a family of MCT proteins (Garcia et al. 1994). MCT1 is widely expressed in different tissues and has been localized in muscle to sarcolemmal and mitochondrial membranes (Brooks et al. 1999; Hashimoto et al. 2005), and facilitates uptake of lactate from interstitium and plasma. The putative role of MCT4 is cellular lactate extrusion. Up to now, Brooks and associates have posited two major lactate shuttles:
- the cell-to-cell lactate shuttle: lactate formed in some muscle cells with high rates of glycolysis can shuttle to other cells with high oxidative capacity via sarcolemmal lactate transporters and be oxidized (Brooks, 1998)
- the intracellular lactate shuttle (ILS): lactate enters mitochondria and is oxidized to pyruvate via mitochondrial lactate dehydrogenase (LDH) when mitochondrial redox decreases, and pyruvate is oxidized via the tricarboxylic acid cycle and electron transport chain (Brooks et al. 1999).
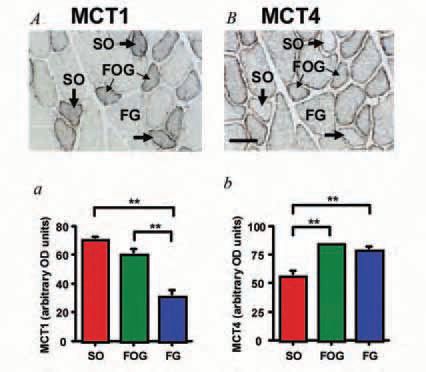
However, the existence of mitochondrial MCT1 and LDH has been controversial. Until our recent paper (Hashimoto et al. 2005), no one had quantified MCT isoform expression by histochemical assessment. Consequently, it was unclear whether MCTs occupied the same or different cell domains in different muscle fibre types. To better understand the physiological roles of MCTs and related proteins, such as mitochondrial inner membrane protein cytochrome oxidase (COX), we sought to determine the distribution and relative abundances of MCTs in rat plantaris that contains different muscle fibre types. Quantitative immunohistochemical determination of MCTs by the high sensitivity avidin-biotin complex (ABC) method showed that MCT1 is located at the sarcolemma and throughout the cell interior in oxidative fibres; in contrast, MCT4 is highly expressed in the sarcolemmal domain of glycolytic fibres (Fig. 1). As well, confocal laser-scanning microscopy (CLSM) demonstrated that MCT1 and COX are co-localized at both interfibrillar and subsarcolemmal cell domains (Fig. 2A). These results show that MCTs and associated proteins are positioned to facilitate function of the lactate shuttles: lactate formed in some muscle cells with high rates of glycolysis (e.g. fast-glycolytic, FG fibres) could be readily released via MCT4 (and MCT1) and transported into slow and fast oxidative (SO and FOG) fibres via sarcolemmal MCT1 lactate transporters. In conjunction, mitochondrial MCT1 facilitates lactate uptake, as oxidation is the major means of intramuscular lactate disposal and therefore essential in establishing the concentration and pH gradients that drive cellular lactate release, uptake and disposal.
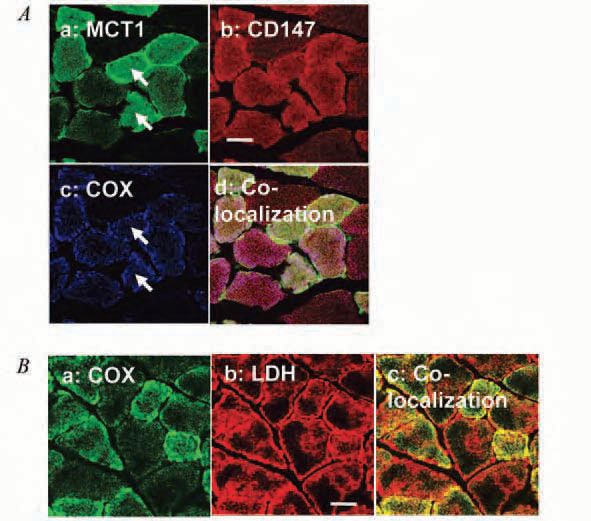
As to the controversy surrounding mitochondrial MCT1 and LDH, we provide strong evidence of their existence. The single-span transmembrane glycoprotein CD147 (BSG, or Basigin) is considered to be the chaperone protein for MCT1 localizing it to the cell surface. Like MCT1 (Fig. 2A-a), we detected CD147 throughout rat plantaris muscle fibres (Fig. 2A-b). Furthermore, MCT1 (Fig. 2A-a), CD147 (Fig. 2A-b), and COX (Fig. 2A-c) were co-localized in individual fibres of rat plantaris muscle (Fig. 2A-d). These findings indicate the existence of MCT1 at mitochondrial inner membrane associated with CD147. Furthermore, we showed the co-localization of COX (Fig. 2B-a) and LDH (Fig. 2B-b) as a yellow colour in Fig. 2B-c, indicating the existence of LDH in mitochondria. Additionally, in cultured L6 skeletal muscle cells, we also observed associations among mitochondrial MCT1, CD147, COX and LDH by both CLSM and immunoprecipitation technique (Hashimoto et al. 2006). Although not yet definitive, results are consistent with presence of a sixth, lactate oxidation, mitochondrial complex.
So, what is the pace of progress? Takeshi Hashimoto learned classic ‘O2 debt’ theory in the late 1990s. Now, checking PubMed there are hundreds of hits for ‘MCT1’ and the ‘lactate shuttle’, where much recent activity has been in the realm of neurobiology. Brooks’ sons Dan and Tim (now 29 and 27 years old) grew up with O2 debt theory in the biology curriculum. But, will Kengo Hashimoto (18 months old) have the same experience?
References
Brooks GA (1998). Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B Biochem Mol Biol 120, 89-107.
Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE & Reeves JT (1991). Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol 71, 333-341.
Brooks GA, Dubouchaud H, Brown M, Sicurello JP & Butz CE (1999). Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A 96, 1129-1134.
Garcia CK, Goldstein JL, Pathak RK, Anderson RG & Brown MS (1994). Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76, 865-873.
Hashimoto T, Masuda S, Taguchi S & Brooks GA (2005). Immunohistochemical Analysis Of Mct1, Mct2 And Mct4 Expression In Rat Plantaris Muscle. J Physiol 567, 121-129.
Hashimoto T, Hussien R & Brooks GA (2006). Colocalization of MCT1, CD147 and LDH in mitochondrial inner membrane of L6 cells: evidence of a mitochondrial lactate oxidation complet. Am J Physiol Endocrinol Metab 290 1237-1244.
Roth DA & Brooks GA (1990). Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279, 377-385.
