
Physiology News Magazine
pH phantoms – a physiological phenomenon?
Recently Christof Schwiening’s lab has shown that fast local pH shifts can occur in nerve cells. But now he is perplexed. The shifts seem to be large, even during modest amounts of electrical activity. Why has no one seen them before? Are they just phantoms? If they are real, are they important or not?
Features
pH phantoms – a physiological phenomenon?
Recently Christof Schwiening’s lab has shown that fast local pH shifts can occur in nerve cells. But now he is perplexed. The shifts seem to be large, even during modest amounts of electrical activity. Why has no one seen them before? Are they just phantoms? If they are real, are they important or not?
Features
Christof J Schwiening
Department of Physiology, University of Cambridge
cjs30@cam.ac.uk / www.physiol.cam.ac.uk/staff/schwiening
https://doi.org/10.36866/pn.50.15
pH and neuronal excitability
Only small changes in pH are required to produce marked alterations in neuronal excitability. It has been known for over 150 years that excessive carbon dioxide acts as a CNS depressant, whilst a lack of carbon dioxide induces hyperexcitability (see references in Somjen & Tombaugh, 1998). Indeed, you can test the effect yourself by first hyperventilating (which lowers carbon dioxide levels in the blood) and then by rebreathing air expired into a paper bag (which raises carbon dioxide levels). Over the past 50 years this link between CO2, pH (see Box 1) and neuronal excitability has been the subject of much experimental work (Tombaugh & Somjen, 1998). Never- theless, I think it is fair to say that most neuroscientists regard pH as irrelevant when considering synaptic mecha- nisms. Of course all experimental electrophysiologists take extreme care with the pH of their patch and bathing solutions – heaven forbid that they are more than 0.1 pH unit out. Experimentally pH is clearly important, but physiologically relevant? Never! For example take an up-to-date review on neuronal synaptic plasticity (Zucker & Regehr, 2002) and look for a mention of pH – there is none. How is it that pH has come to be so ignored?

Intracellular pH regulation
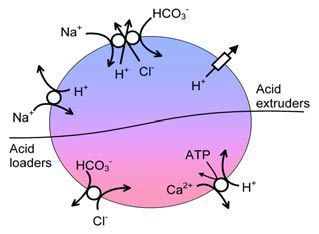
The downfall of pH lies partly in its very importance. Since the rates of all enzyme reactions are sensitive to pH, but to different degrees, it has long been supposed that pH must be under extremely tight control. Were this not to be the case then a metabolic mess would occur, with some reactions proceeding too fast and others too slowly. The result of this mess would be a decline in normal physiological function. This view of pH homeostasis is supported by the exquisite control our bodies place on blood pH through the modulation of breathing. Furthermore, the fact that almost all cells maintain intracellular pH (pHi) about 1 pH unit more alkaline than would be expected from passive diffusion of H+ (or indeed H3O+ or OH-) (*1) across the plasma membrane supports this idea of active pH regulation. Thus, the view now commonly held is that pH, at least of nerve cells, is stabilized at an optimum level by pH regulating mechanisms (Figure 1).
It was with the study of pH regulation that my interest in neuroscience began. As a PhD student I impaled locust neurones, generally unsuccessfully, with ion-sensitive microelectrodes in an attempt to discover the mechanisms that regulated pH. At the time the idea generally held was that the gradual leak of H+ into cells, down its electrochemical gradient, and metabolic H+ production represented the H+ load that the regulating mechanisms tirelessly fought. To investigate pH regulation it was deemed necessary to perturb pH by the addition of exogenous acid and study the ionic dependence of the subsequent pH recovery (Thomas, 1984). It was already known that snail neurones and squid giant axons regulate pH with a plasma- membrane Na+-dependent Cl-/HCO3 -exchanger (Figure 1). To many in the department in Bristol my study on locusts was esoteric and rather dull. Maybe they had a point.
During my first post-doc, in the USA, I saw others trying to make the study of pH both exciting and trendy. I was introduced to a phenomenon we called the ‘ugly monster’, a pH change resulting from the activation of an extracellular solute receptor. I was also set to work on the intense activation of acid extrusion in hippocampal neurones, which I was told might underlie memory! Both, unfortunately for me, turned out to be artefacts. Finally, I got involved in an expression cloning project, but it came to nothing. I left after a year, wary of both artefacts and molecular biology. As I travelled back across the Atlantic no doubt there was a collective sigh of relief from the lab!
Activity dependent pH changes
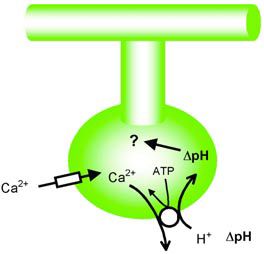
In 1992 I was being funded by the MRC to look at calcium buffering in snail neurones (a model nerve cell). I was failing to measure calcium levels with calcium-sensitive microelectrodes whilst Helen Kennedy was having problems persuading colleagues that snail neurones showed no sign of a plasma-membrane Na+/Ca2+ exchanger. At the time it was widely believed that neurones extruded the bulk of their calcium on the Na+/Ca2+ exchanger. Roger Thomas, on the other hand, was having plenty of luck measuring extra- cellular surface pH shifts. I wondered if I could measure surface calcium changes. By lowering extracellular calcium to about 1/50th of its normal level I was able to see calcium disappearing from the external surface during depolarisation (presumably entering the cell through voltage-gated calcium channels) and then gradually re-emerging following repolarization. Removing external Na+ did not prevent the reappearance of calcium, but the intracellular injection of vanadate (an inhibitor of ATP-dependent pumps) did. This seemed to implicate the calcium pump in snail neurone calcium extrusion. Furthermore, after a month of frenetic experiments, I managed to show that the calcium extrusion was coupled to the inward transport of H+ and that it was the calcium pump that gave rise to the extracellular alkaline shifts that Roger had been recording. We were very excited by this discovery of the counter-transport of H+ on the plasma membrane calcium pump (PMCA; also known as the Ca2+-ATPase or calcium-hydrogen pump) since it seemed to us that it might explain a whole host of pH changes in the CNS. Nature was, however, not impressed – and who can blame them, after all this was work from snails – and the work was published in an austere but august journal (Schwiening, et al 1993). Now I believe it is reasonably well accepted that some of the pH shifts seen in the vertebrate CNS do indeed arise from the counter-transport of H+ on the PMCA (Trapp et al 1996).
Of course those working on red blood cells had known for some time that their PMCA could transport protons – but this was considered by them an irrelevance in a system with little calcium permeability and oodles of other H+ flux (see Box 2). It is a shame that for 10 years those working on neuronal pH regulation were seemingly unaware of the RBC literature.

Armed with a Wellcome Trust Career Development Fellowship I then set about looking at activity-dependent pH shifts in neurones.
BCECF v HPTS
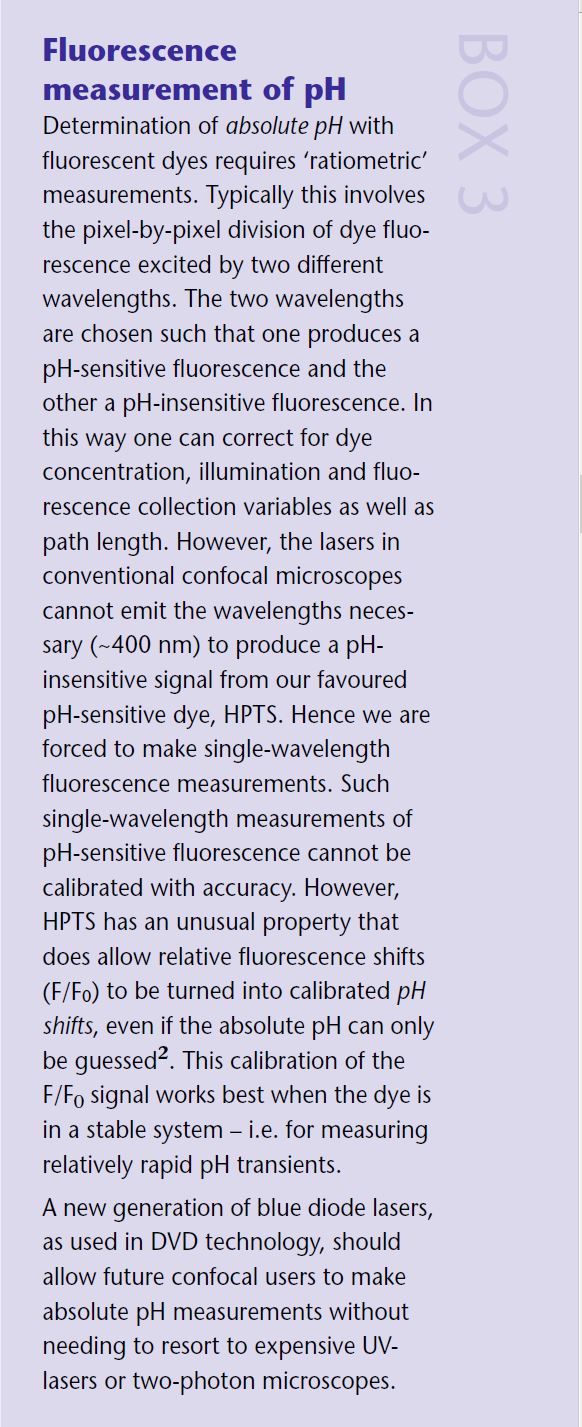
My initial experiments using a pH- sensitive dye called BCECF failed all too frequently. This fluorescein-based dye was hard to load into snail neurones through the patch pipette. The pipettes frequently blocked and my signals were buried in the noise. At that point I began to try out other pH-sensitive dyes. I found a cheap dye (£15 per gram, of which I still have some left) called HPTS which seemed to perform excellently, although few others used it. Presumably its lack of popularity was because it was not available in a membrane-permeant form. To my horror I also discovered, from the red blood cell literature, that BCECF was a potent inhibitor of the PMCA! (see Box 2) Most workers measuring pH in neurones, at the time, seemed unaware of both the blocking effect of BCECF on the PMCA and the link between the PMCA and pH. This was to some extent reasonable since, at the time, it was assumed that most neurones had little PMCA activity. My experiments with the non-blocking pyranine-based pH-sensitive dye HPTS and a potent blocker of the PMCA, eosin, seemed to show that the PMCA was the main endogenous acid loader in snails. When the PMCA was blocked the calcium-dependent pH shifts temporarily disappeared before reappearing possibly due to mitochondrial dumping of acid. In 1996, with another two years of my Fellowship to go, I took up a lectureship in Cambridge.
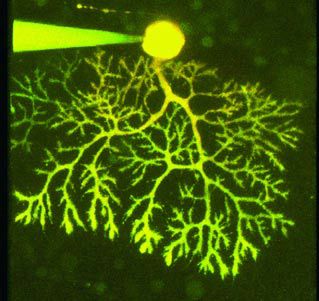
My first attempts to measure regional pH in neurones were made using a fluorescence imaging system and freshly isolated rat hippocampal neurones. It was, frankly, a disaster from start to finish. Then after those three frustrating years with few results but many ideas, the MRC awarded us with a de novo Co-Operative Group Grant. This included the funds to buy a confocal microscope and investigate, amongst other things, pH shifts in snail neurones and rat cerebellar Purkinje neurones (Figure 2).
Confocal microscopy in Cambridge
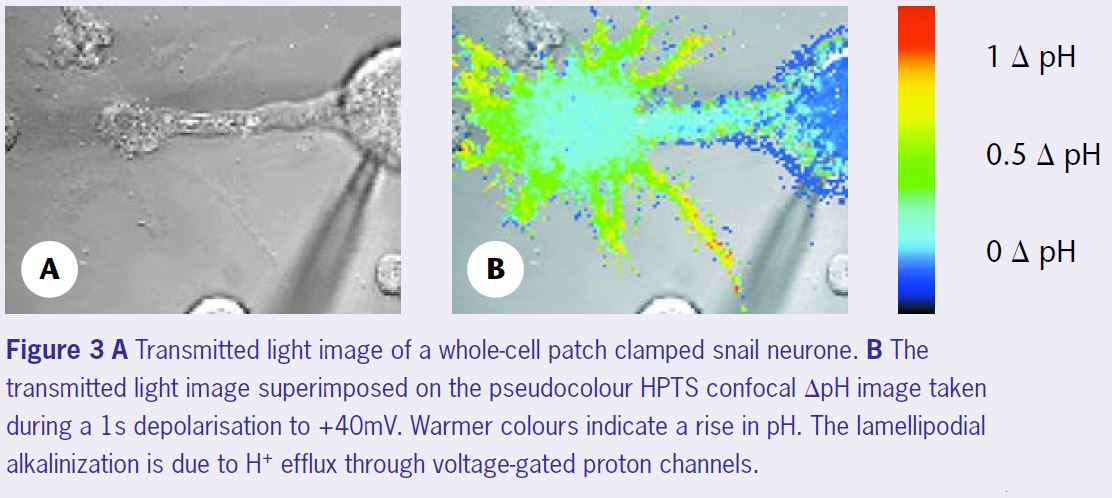
To make spatial recordings of pH we (Debbie Willoughby and I) planned to image the pH-sensitive fluorescence of HPTS (Willoughby et al 1998) on the confocal microscope. Because the confocal scans a beam of light across the preparation, rather than illuminating it all in one go, the very bright cell body fluorescence does not (necessarily) obscure the weak dendritic and axonal signals. At the time, neither of us had had any experience of confocal microscopy and we were given the impression that there were many obstacles ahead. Indeed, the signals we obtained from the processes were small, less than a hundredth of those that could be got from the soma. Further- more, since the diffusion of the dye, from the patch-pipette to the processes, was relatively slow we had to be careful not to ‘bleach’ too much of the dye. But, since we could use quite a high concentration of the dye, these turned out to be relatively minor problems. One of the great advantages of HPTS, over most of the fluorescein-based dyes, is that when excited with either the 488 nm or 458 nm line of the Argon ion laser the entire fluorescence is pH sensitive – there is no pH-insensitive ‘pedestal’ fluorescence. This allows the single wave- length fluorescence shifts to be calculated without the need for any laborious calibrations. (*2) To our knowledge this was the first time that HPTS had been used on a confocal to follow regional pH, and it took quite a time before we felt confi- dent with the measurements we were making. Figure 3 shows such a calibrated ΔpH image of a snail neurone during a depolarisation to +40mV. The lamellipodia are clearly subjected to much larger alkaline pH shifts than the cell body. Although some had predicted that such pH gradients must exist in neurones (eg Tombaugh, 1998), seeing them directly was very exciting.
Rat Purkinje neurones
The choice of cerebellar Purkinje cells for our mammalian neuronal preparation was fortuitous. They turned out to be ideal for confocal imagery and easy to patch. The data analysis was, however, more of a challenge and led me to write a little bit of software (see my home page if you want a copy!) to perform regional pixel-by-pixel F/F0 plots (see Box 3). Characterizing the pHi shifts was also not simple. The Purkinje cells are architecturally complex and attempting to control membrane potential throughout the dendritic ramifications with the patch pipette is almost certainly a lost cause.
In the end blocking inhibitory input with bicuculline and dialyzing with Cs+ (to block K+ channels) turned out to be about the best we could do for voltage clamp recordings. But, as you can see from the prolonged dendritic calcium trace in Figure 4, this tended to result in the dendritic regions ‘hanging up’ at positive potentials for rather longer than the cell body. Thus we also used trains of back propagating action potentials (again with bicuculline present, but no Cs+). The results were very similar in both cases. Electrical activity, even in the presence of physio- logical amounts of CO2/HCO3 – and carbonic anhydrase activity, caused dendritic pH shifts (~0.1 pH unit for either 1 s depolarisation or a 10 s burst of action potentials) that were about three times larger than that seen in the cell body. By combining a calcium- sensitive dye (Fura-red) with our pH- sensitive dye we began to look at whether these heterogeneous pH transients occur as a result of differences in the calcium transients, or whether there might be other factors at work, such as local pH regulation.
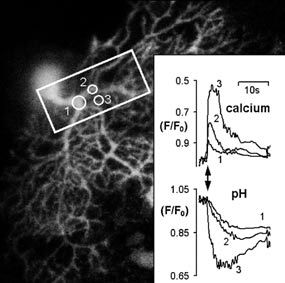
Of course, there are many questions remaining. Although we know that these pH shifts are dependent upon calcium influx, we do not know what is causing them. We assume that it is the PMCA, but it could equally result from the uptake of calcium into internal stores (mitochondria or endoplasmic reticulum). We do not know whether pHi is uniform across the Purkinje cell at ‘rest’. If the dendritic spines are the sites of H+ influx into the cytosol, it is possible that the pH shifts there might be much larger (and indeed faster). Then we come to the question: do these pH shifts really occur physiologically? After all these are brain slices where the blood supply has been removed, they are at room temperature, the cells are being dialyzed with a patch pipette solution and artificially stimulated. This is far from physiology. But imaging pH in nerve cells in a functioning brain during physiological stimulation (eg tickling a paw) takes more money than the MRC can currently pay and more time than the University, driven by research assessment objectives, will allow. But, we can dream!
If we cannot perform the experiment, can we at least muse on the possible roles of these pH shifts? There are a couple of pieces of evidence that might suggest that they are important in normal physiology. Both sets of experiments involve modifying pH buffering. Firstly, in experiments on isolated hippocampal neurones, it has been shown that increasing the concentration of intracellular pH buffer can modify neuronal calcium signals. (Tombaugh, 1998). The introduction of excess pH buffer into cells should not directly alter pHi. It has been suggested that the pHi shifts that normally occur during electrical activity (Trapp et al 1996) might be blunted by the additional buffer. However, in his study, Tombaugh was unable to make recordings of these dendritic pH shifts. The second set of experiments, or rather observations, are from human clinical studies.
Cerebellar ataxia
Certain types of CNS disorders can be treated with inhibitors of carbonic anhydrase (such as acetazolamide). Such inhibition is well known to magnify the size of activity-dependent pH shifts. Amongst these disorders are two that are known to involve mutations of the P/Q-type calcium channels (hemiplegic migraine and episodic ataxia type II; see review by Kullmann, 2002). The clinical effectiveness of acetazolamide has been assumed to be due to an effect on resting pHi. However, given that the disorders are associated with impaired calcium influx during depolarization – and therefore presumably smaller calcium- dependent pHi shifts – it is more likely that acetazolamide is simply restoring the otherwise blunted pHi shifts (Figure 5). Such a hypothesis, of course, relies on the assumption that pHi shifts play an important role in normal neuro- physiology and that their absence results in a profound dysfunction. This is not what most neuroscientists currently appear to think.
Whither?
At this point it is not clear that we know the full extent of these local pH signals since they have not yet been measured in locations where they are likely to be largest; pre-synaptic terminals and dendritic spines. Nevertheless, the investigation of the physiological (and indeed clinical) importance of these pH shifts seems worth some effort.
However, we have few tools (eg blockers) with which to study these functions. In order to remove the pH signals it is currently necessary to abolish the transmembrane calcium fluxes – hardly a specific blocker! Manoeuvres designed to enhance the pH shifts have little to add to our knowledge since they might only reveal what the physiological buffer systems may normally attempt to prevent.
To restrict our interest to neurones would also be a mistake. There must be researchers working on non- neuronal cells for whom local pH shifts could also be important. Preparations in which either large calcium fluxes and concomitant high levels of PMCA activity are known to occur (eg cochlear hair cells) or where moderate calcium fluxes occur in regions with little cytoplasmic volume (eg ciliated portions of olfactory receptors) are both likely to show local pH shifts. Finally, there is also mounting evidence that local pH shifts might regulate calcium fluxes during calcium release from stores. This is something that is currently making our MRC Co-op twitch.
*Notes
1: I use H+ here to represent an acid equivalent, of course it could equally be H3O+, H5O2+ and so on. Furthermore a move- ment of H+ in one direction could equally be a movement of OH- in the other direc- tion. For the sake of this article such differences should cause us little worry.
2: It does, however, require knowledge of the pK of HPTS and the approximate starting pHi. Of these two variable the starting pH is least certain, however the calibration is relatively insensitive to pH at neutral and acidic values. The formula is: ΔpH = log (F/F0) – log (1-(F/F0-1).10(pH-pK) )
References
Gatto C & Milanick MA (1993). Inhibition of the red blood cell calcium pump by eosin and other fluorescein analogues. Am J Physiol 264, C1577- 1586.
Kullmann, DM (2002). The neuronal channelopathies. Brain 125, 1177-1195.
Niggli V, Sigel E & Carafoli E (1982). The purified Ca2+ pump of human-erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem 257, 2350-2356.
Rossi JPFC & Schatzmann HJ (1982). Is the red cell calcium pump electrogenic? J Physiol 327, 1-15.
Schwiening CJ, Kennedy HJ & Thomas RC (1993). Calcium-hydrogen exchange by the plasma membrane Ca-ATPase of voltage-clamped snail neurons. Proc R Soc Lond 253, 285-289.
Schwiening CJ & Willoughby D (2002). Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol 538, 371-382.
Somjen GG & Tombaugh GC (1998). pH modulation of neuronal excitability and central nervous system fuctions In pH and brain function, (ed. Kaila K & Ransom BR), pp. 373-394. New York: Wiley-Liss.
Thomas RC (1984). Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol 354, 3P-22P.
Tombaugh GC (1998). Intracellular pH buffering shapes activity-dependent Ca2+ dynamics in dendrites of CA1 interneurons. J Neurophysiol 80, 1702-1712.
Tombaugh GC & Somjen GG (1998). pH modulation of voltage-gated ion channels. In pH and brain function, (ed. Kaila K & Ransom BR), pp. 395-416. New York: Wiley-Liss.
Trapp S, Luckermann M, Kaila K. & Ballanyi K.(1996). Acidosis of hippocampal-neurons mediated by a plasmalemmal Ca2+/H+ pump. NeuroReport 7, 2000-2004.
Willoughby D. & Schwiening CJ (2002). Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol 544, 487-499.
Willoughby D, Thomas RC & Schwiening CJ (1998). Comparison of simultaneous pH measurements made with 8-hydroxypyrene-1,3,6- trisulphonic acid (HPTS) and pH-sensitive microelectrodes in snail neurones. Pflügers Arch 436, 615-622.
Zucker RS & Regehr WG (2002). Short-term synaptic plasticity. Annu Rev Physiol 64, 355-405.
