
Physiology News Magazine
Physiological Control of Behaviour at Low Temperatures
In this article John Young discusses the relationship between severe low temperature and neuronal activity and goes on to suggest that an axonal equivalent to a warm overcoat may just be the key.
Features
Physiological Control of Behaviour at Low Temperatures
In this article John Young discusses the relationship between severe low temperature and neuronal activity and goes on to suggest that an axonal equivalent to a warm overcoat may just be the key.
Features
John S Young
Department of Zoology University of Cambridge
https://doi.org/10.36866/pn.49.18

Temperature affects the rate of every physiological process. As low temperatures are experienced by animals from virtually all environments, animals that cannot regulate their body temperature – poikilotherms – must have mechanisms to survive low temperatures, and most of these survival mechanisms are well documented. Animals not only need to survive low temperatures, but some must also be able to produce rapid behaviours; they must have evolved compensation for the thermodynamic tendency of processes to slow down in the cold. These physiological adaptations that allow the production of rapid behaviours in poikilotherms living at low temperatures are less well understood. In my PhD I am investigating the function of the nervous system in marine crustaceans from temperate and polar environments.
Surviving low temperatures
Animals adopt different mechanisms to deal with low temperature depending on the duration of the cold period. Animals that experience low temperatures only transiently deal with the formation of potentially damaging ice crystals using mechanisms of freeze tolerance: nucleators control the formation of ice crystals in extracellular spaces, while cellular adaptations minimise cell shrinkage and ensure a rapid distribution of cryoprotectant compounds that lower the freezing point of body fluids. This enables freeze tolerant springtails (a six-legged hexapod), Gomphio-cephalus hodgsoni, to survive temperatures as low as -38°C (Sinclair and Sjursen 2001; freeze tolerance reviewed by Storey and Storey 1996).
During the Antarctic summer, terrestrial species must be able to survive rapid drops in temperature. The Antarctic springtail, Cryptopygus antarcticus, is able to survive rapid cooling over a 20h period from +5 to -5°C. This tolerance was originally believed to be the result of gut clearance, (thereby removing particles that could act as unwanted ice nucleators) and altering the concentration of body fluids, but Worland and Convey (2001) show that these mechanisms are not used.
Animals from permanently frozen environments use mechanisms of freeze avoidance to depress the point at which damaging ice crystals form in body fluids. Compounds that could act as ice nucleators are removed, polyols are accumulated and an external waterproofing prevents contact with environmental ice, the most potent of nucleators (reviewed by Zachariassen and Kristiansen 2000).
Maintaining behavioural activity
Animals that experience transient low temperatures generally show a reduction in behavioural activity and metabolic rate as temperature decreases. Over seasonal time scales they either become dormant, or continue to function normally with compensated phys ological rates. Animals from high latitude or high altitude environments experience low temperatures permanently and therefore must remain active. The Alaskan beetle, Pterostichus brevicornis, shows normal behavioural activity at temperatures as low as –12°C, at which closely related temperate species could not survive(Baust 1972).
Maintained rates of behavioural output are believed to arise from the principle of metabolic cold adaptation: which states that physiological rates should be equal in polar, temperate and tropical poikilotherms. An animal living in a low temperature environment is said to be cold-adapted if its metabolic rate is greater than that of a related temperate species cooled to the same temperature. The extent of cold adaptation depends on the extent of temperature fluctuations. There is little temperature compensation of metabolic processes in Antarctic marine invertebrates (e.g. Peck and Conway 2000), suggesting that aerobic capacity and energy expenditure is minimised and tuned to the stable marine environment. Whether terrestrial poikilotherms from permanently freezing environments show similarly low (uncompensated) metabolic rates, compared to related temperate species, is contentious. High rates of oxygen consumption between 5 and 10°C in a sub-Antarctic dipteran, Paractora dreuxi, suggest a rapid acceleration of metabolism as temperature rises after a cold spell (Crafford and Chown 1993), representing a metabolic adaptation to ensure maximum gains during periods of productivity. In contrast no evidence of metabolic cold adaptation was found in Arctic arthropods (Scholander et al 1953). This is believed simply to represent a cost-saving mechanism, allowing resources to be devoted to growth and development. The true story is confused because latitudinal differences in metabolic rates are prone to species- bias, and because of variations in the methodology used.
There are certainly mechanisms of cellular adaptation over seasonal and evolutionary time scales, but whether they aid compensated rates of physiological processes or simply allow survival is not known. It has been well documented that lipids become more solid with decreasing temperature, so to compensate, membrane fluidity is altered to maintain cellular function, with saturated fatty acid moieties substituted for their unsaturated equivalents (e.g. Clarke 1977).
Relating the viscosity of neuronal membranes to behavioural performance over a range of temperatures (e.g. Cossins et al 1977) led many to believe that it was key changes in fatty acid composition over seasonal or evolutionary timescales that allowed some species to produce rapid behaviours at subzero temperatures.
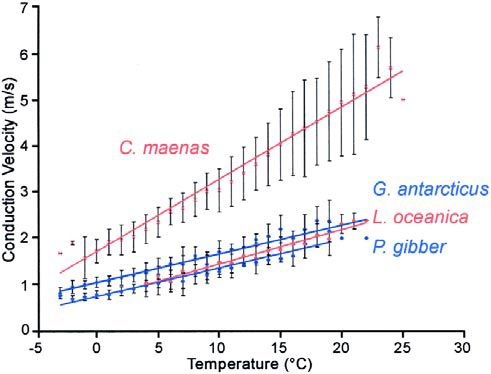
Subsequent comparisons of closely related species from very different thermal environments have revealed other structural adaptations which permit occasional rapid behaviour in low temperature environments. In my PhD, I am assessing the effect of temperature on the neuronal control of behaviour in four marine crustacean species from very different thermal environments: the crab, Carcinus maenas, and the isopod, Ligia oceanica, from the British coastline, where the temperature varies between +2 and 24°C annually, and the giant isopod, Glyptonotus antarcticus, and the amphipod, Paraceradocus gibber, from Antarctica, where the sea temperature is –1.8±1°C.
There were positive relationships between temperature and neuronal conduction velocity in the leg nerves of all four species with propagation maintained up to 20°C (figure 1). This is particularly interesting as the Antarctic species do not survive temperatures above 6°C, supporting the hypothesis proposed by Prosser (1973) that other neuronal functions are more sensitive to temperature change than is axonal conduction. There is a difference between closely related species from different thermal environments, however; axonal conduction velocity was more rapid at any given temperature in G. antarcticus than in L. oceanica (ANCOVA F1,139 = 29.52, p<0.001), suggesting some form of neuronal compensation.
A neuroanatomical basis for this finding was investigated using transmission electron microscopy of the same nerve. Although L. oceanica has a greater percentage of large (>20mm) axons than G. antarcticus, the large axons in the latter species had thick (5 µm) wrappings (figure 2). Experiments to determine the composition of these wrappings, and whether they increase the speed of axonal conduction, are still to be performed.

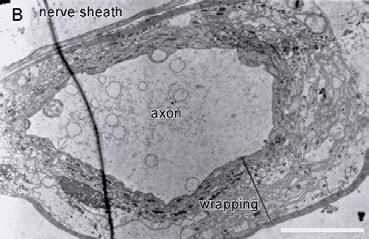
The hypothesis that the wrapped axons of the Antarctic isopod, G. antarcticus, may serve to increase the speed of neuronal conduction, is supported by similar findings in other taxa. Alpine crickets (Morrissey and Edwards 1979; Edwards and Mann 1981) produce rapid bouts of walking over large distances at near-freezing temperatures along a surface of snow and ice. The closely related house cricket is torpid at the same temperature. This behavioural adaptation is explained by wrapped axons in the alpine species which are shown to continue propagating action potentials down to -3.5°C whereas axonal conduction ceases at +4°C in the house cricket.
There are few other examples of compensations that counteract the tendency for motor functions to decrease in rate in low temperature environments. For many species that experience permanent low temperatures, particularly Antarctic marine invertebrates, reduced behavioural activity represents the most energy efficient way of coping with low food availability. These animals are unlikely to show neurophysiological compensation. The capacity to produce rapid behaviours may be reserved to a few species that either need to evade predation or scavenge for food. Such species include a few Antarctic marine invertebrates, such as G. antarcticus, Antarctic marine vertebrates and alpine invertebrates. How the neurophysiological control of rapid behaviours is adapted for low temperatures is not known. Future experiments will determine what other adaptations allow rapid behaviour at both low and fluctuating temperature. Many questions remain, including what role does the nervous system play in governing the flexibility of an animal to tolerate changes in body temperature? Are the adaptations that allow rapid axonal conduction at low temperatures also responsible for the very narrow temperature range over which Antarctic invertebrates can produce coordinated behaviour?
By addressing these questions I hope that we can better our understanding of the flexibility of the physiological control of behaviour of animals living in low temperature environments.
Acknowledgements
I thank Lloyd Peck, Keri-Lee Page and my supervisor Tom Matheson for their valuable comments on this manuscript. This work is supported by a studentship from the BBSRC. I would like to thank Professor Lloyd Peck from the BAS, and the National Science Foundation for the supply of animals.
References
Baust, J.G.(1972) Temperature-induced neural adaptations: motoneuron discharge in the Alaskan beetle Pterostichus brevicornis (Carabidae). Comp Biochem Physiol A 41: 205-213
Clarke, A. (1977) Lipid class and fatty acid composition of Chorismus antarcticus (Pfeffer) (Crustacea: Decapoda) at South Georgia. J Exp Mar Biol Ecol 28: 297-314
Cossins, A.R., Friedlander, M.J. and Prosser, C.L. (1977) Correlations between behavioural temperature adaptations of goldfish and viscosity and fatty acid composition of their synaptic membranes. J Comp Physiol 120: 109-121
Crafford, J.E. and Chown, S.L. (1993) Respiratory metabolism of sub-Antarctic insects from different habitats on Marion Island. Polar Biol 13: 411-415
Edwards, J.S. and Mann, D. (1981) The structure of the cercal sensory system and ventral nerve cord of Grylloblatta. Cell Tiss Res 217: 177-188
Morrissey, R. and Edwards, J.S. (1979) Neural function in an alpine grylloblattid: a comparison with the house cricket Acheta domesticus. Physiol Entomol 4: 241-250
Peck, L.S. and Conway, L.Z. (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. In: Harper, E.M., Taylor, J.D. and Crame, J.A. (eds) The evolutionary biology of the Bivalvia. Geological Society, London, Special Publications 177: 441-450
Prosser, C.L. (ed.) (1973) Comparative animal physiology. Saunders, Philadelphia Scholander, P.F., Flagg, W., Walter, V. and Irving, L. (1953). Climatic adaptation in Arctic and tropical poikilotherms. Physiol Zool 26: 67-92
Sinclair B.J. and Sjursen H. (2001) Cold tolerance of the Antarctic springtail Gomphiocephalus hodgsoni (Collembola, Hypogastruridae). Ant Sci 13 (3): 271- 279
Storey, K.B. and Storey, J.M. (1996) Natural freezing survival in animals. Ann Rev Ecol Syst 27: 365-386
Worland, M.R. and Convey, P. (2001) Rapid cold hardening in Antarctic microarthropods. Funct Ecol 15: 515-524
Zachariassen K.E. and Kristiansen E (2000) Ice nucleation and antinucleation in nature. Cryobiology 41 (4): 257-279
