
Physiology News Magazine
Pulsed infrared radiation: a potential tool to reveal cellular secrets
Pulsed infrared radiation (IR) has been shown to stimulate excitable cells through activating key intracellular signalling events. Data suggest the presence of fundamental IR-sensitive mechanisms that generalize across cell types. Our recent results demonstrate the importance of IR-modulated intracellular Ca2+ signalling in cardiomyocytes and inner-ear hair cells. Application of pulsed IR to modulate intracellular Ca2+ may be particularly useful to study events controlling synaptic transmission and excitation–contraction coupling.
Features
Pulsed infrared radiation: a potential tool to reveal cellular secrets
Pulsed infrared radiation (IR) has been shown to stimulate excitable cells through activating key intracellular signalling events. Data suggest the presence of fundamental IR-sensitive mechanisms that generalize across cell types. Our recent results demonstrate the importance of IR-modulated intracellular Ca2+ signalling in cardiomyocytes and inner-ear hair cells. Application of pulsed IR to modulate intracellular Ca2+ may be particularly useful to study events controlling synaptic transmission and excitation–contraction coupling.
Features
Suhrud M. Rajguru (1,2) and Richard D. Rabbitt (3,4)
1: Department of Otolaryngology, Northwestern University, Chicago, IL, USA
2: Department of Biomedical Engineering, University of Miami, Miami, FL, USA
3: Marine Biological Laboratory, Woods Hole, MA, USA
4: Department of Bioengineering, University of Utah, Salt Lake City, UT, USA
https://doi.org/10.36866/pn.84.25


Arsonval (1891) was the first to demonstrate endogenous photosensitivity of cells. It was later recognized that the responses to ‘light’ are widespread and even occur in cells that are normally shielded from optical radiation. For example, it has been known for five decades that optical radiation can evoke action potentials in neurons (Arvanitaki & Chalazonitis, 1961), and that optical responses can be exploited to identify functional connections in neural networks. Since the invention of the laser, radiations of various wavelengths have been used to irradiate and stimulate neurons. The primary mechanism of action in these early studies was thermal responses of ion channels and the heat deposited by optical radiation. The non-specific thermal origin of responses dampened broad use of optical stimuli. The discovery of direct light-activated ion channels (Nagel et al. 2002) and the development of techniques for photocontrol of neurons in vivo (Boyden et al. 2005) has provided a new set of optogenetic tools for photo-excitation and -inhibition of cells independent of heat. But heat itself can be a powerful stimulus. New laser and optical technologies have enabled the delivery of very short focused pulses of IR, a stimulus that delivers transient pulses of thermal energy (dT/dt) without large increases in accumulated temperature of cells or tissue. Pulsed IR activates endogenous physiological mechanisms within the cell, and the mechanisms may vary based on the heterogeneous absorption spectra of the key molecules expressed. IR stimuli have been successfully applied for neuroprostheses (Wells et al. 2005; Rajguru et al. 2010), cardiac pacemaking (Jenkins et al. 2010) and studies of intracellular signalling (Dittami et al. 2011).
Wells et al. (2005) applied pulsed IR to evoke action potentials in regions of the rat sciatic nerve to elicit contraction of distinct muscle groups. Pulsed IR has been used to stimulate the facial (Teudt et al. 2007) and auditory nerves (Izzo et al. 2006). Although it is clear that pulsed IR delivers transient kinetic energy to molecules comprising the cell, which specific molecules are responsible for excitability has been a subject of debate and probably varies between cell type, location of the radiation and the wavelength. Transient receptor potential channels (TRPs), stretch-activated channels and biochemical kinetics (e.g. Arrhenius model) may all be involved. It is clear that IR applied to the cell body or synapse modulates intracellular Ca2+ ([Ca2+]i). We have reported evidence in rat neonatal cardiomyocytes that 1862 nm pulsed IR activates mitochondrial Ca2+ flux and that blocking the mitochondrial Ca2+ uniporter (mCU) or the mitochondrial Na+–Ca2+ exchanger (mNCX) blocks the major component of IR excitability in these cells (Dittami et al. 2011). Assuming that this IR-modulated Ca2+ signalling is a general principle, we expected the inner-ear hair cells to also be sensitive to IR due to their high mitochondrial density and the Ca2+-dependent synaptic machinery. In the hair cells, intracellular Ca2+ plays an important role in signal transduction. Increases in intracellular Ca2+ have been shown to be involved in several important processes in mammalian hair cells, including adaptation of the mechanotransducer current and in triggering neurotransmitter release.
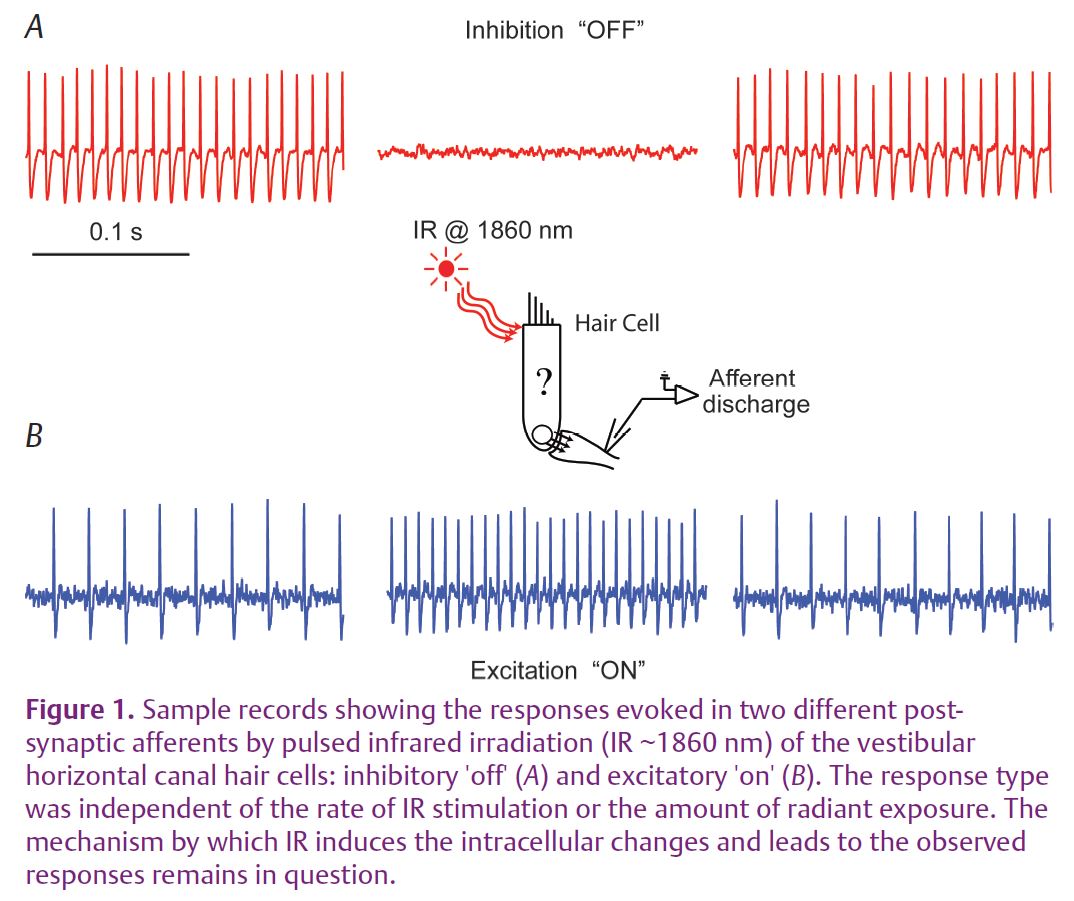
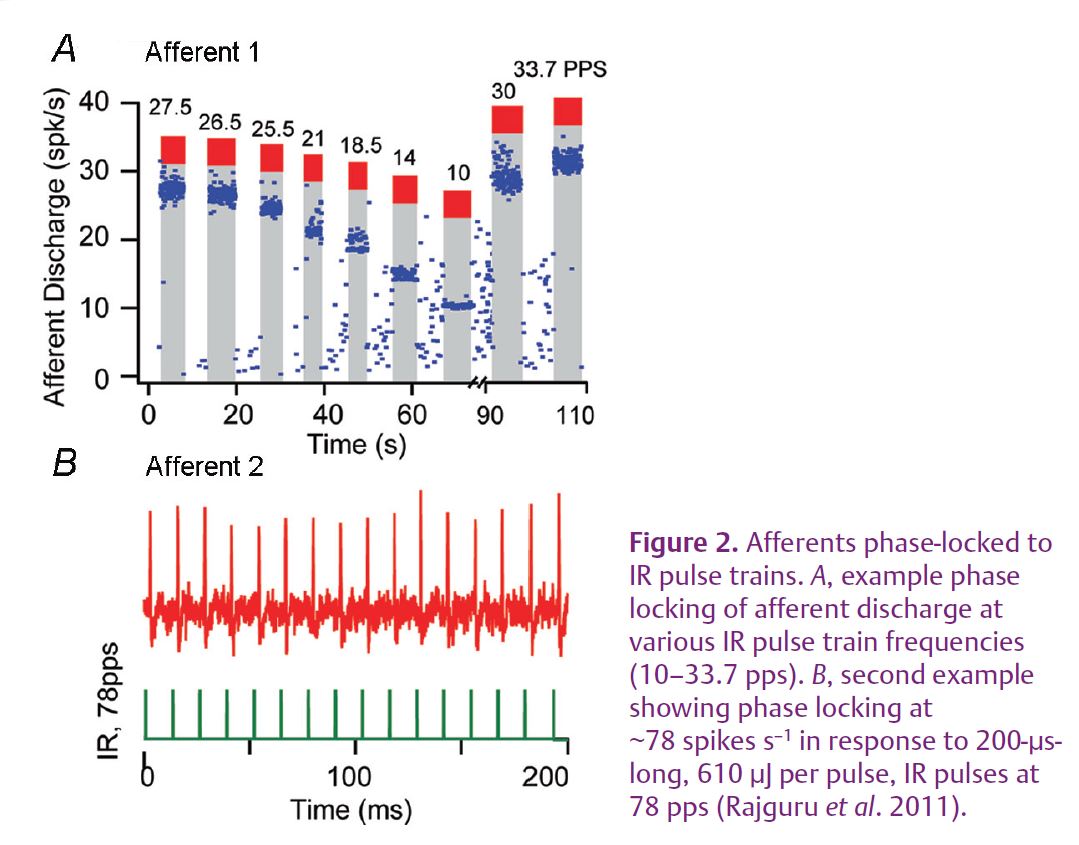
In a recent study published in The Journal of Physiology, we applied pulsed IR (1860 nm, pulse durations ranging from 200 to 750 µs and with pulse repetition rates of up to 100 Hz) to stimulate presynaptic hair cells in the vestibular organs of the toadfish, Opsanus tau, and recorded evoked responses from the post-synaptic afferent neurons in vivo (Rajguru et al. 2011). The radiation was delivered to the vestibular epithelium using a 200 or 400 µm optical fibre coupled to a diode laser. This non-contact method allowed stimulation and neural recordings to be carried out for many hours. IR stimulation of hair cells resulted in robust single-unit semicircular canal afferent responses (Fig. 1). Some afferent neurons were excited, some were inhibited, and some showed a mixed inhibitory–excitatory pattern. Afferents that were tested for phase-locking to pulsed IR up to 100 Hz, responded pulse-by-pulse with an action potential (Fig. 2). The responses of the post-synaptic afferents did not diminish after many hours of stimulation. Responses were not due to overall changes in accumulated temperature of the sensory organ, but probably were evoked by heterogeneous changes in transient molecular thermal kinetic energy imparted by the IR. IR pulse-by-pulse evoked responses suggest that the transient energy relaxed prior to arrival of the subsequent IR pulse. Also, the IR-evoked responses persisted when the whole-body temperature was reduced to the extent expected to close key TRP channels. In rat neonatal cardiomyocytes, IR evoked intracellular [Ca2+]i transients, distinct from spontaneous [Ca2+]i events and persisted after blocking the Ca2+-activated Ca2+ machinery of the cell. These results raise the possibility of using pulsed IR to study certain events related to excitation–contraction coupling and synaptic transmission that are difficult to examine using conventional approaches.
Recent results using pulsed IR stimuli clearly demonstrate its potential for basic research and suggest promise for the future development of therapeutic interventions. Realizing the full potential of IR stimuli, however, remains limited by inadequate characterization of stimulus-dependent cellular/molecular mechanisms.
References
Arsonval AD (1891). La fibre musculaire est directement excitable par la lumiere. CRSoc Biol 43, 318–320.
Arvanitaki A & Chalazonitis N (1961). Excitatiory and inhibitory processes initiated by light and infra-red radiations in single identifiable nerve cells. In Nervous Inhibition, ed. Florey E. Pergamon Press, New York.
Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8, 1263–1268.
Dittami GM, Rajguru SM, Lasher RA, Hitchcock RW & Rabbitt RD (2011). Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J Physiol 589, 1295–1306. http://jp.physoc.org/content/589/6/1295.long
Izzo AD, Richter C-P, Jansen ED & Walsh JT Jr (2006). Laser stimulation of the auditory nerve. Lasers Surg Med 38, 745–753.
Jenkins MW, Duke AR, Gu S, Chiel HJ, Fujioka H, Watanabe M, Jansen ED & Rollins AM (2010). Optical pacing of the embryonic heart. Nat Photonics 4, 623–626.
Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E & Hegemann P (2002). Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398.
Rajguru SM, Matic AI, Robinson AM, Fishman AJ, Moreno LE, Bradley A, Vujanovic I, Breen J, Wells JD, Bendett M & Richter CP (2010). Optical cochlear implants: evaluation of surgical approach and laser parameters in cats. Hear Res 269, 102–111.
Rajguru SM, Richter CP, Matic AI, Holstein G, Highstein SM, Dittami GM & Rabbitt RD (2011). Infrared photostimulation of the Crista Ampullaris. J Physiol 589, 1283–1294. http://jp.physoc.org/content/589/6/1283.long
Teudt IU, Nevel A, Izzo AD, Walsh jT Jr & Richter CP (2007). Optical stimulation of the facial nerve – a new monitoring technique? Laryngoscope 117, 1641–1647.
Wells J, Kao C, Jansen ED, Konrad P & Mahadevan-Jansen A (2005). Application of infrared light for in vivo neural stimulation. J Biomed Opt 10, 064003.
