Physiology News Magazine
Reactive oxygen species and glucose transport during exercise
Reactive oxygen species and glucose transport during exercise
Marie E Sandström, Shi-Jin Zhang, Joseph Bruton, José P Silva (1), Michael B Reid (2), Håkan Westerblad, Abram Katz
Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden
1: Laboratory of Metabolic Diseases, The Rockefeller University, New York, NY, USA
2: Department of Physiology, University of Kentucky Medical Center, Lexington, KY, USA
https://doi.org/10.36866/pn.66.16
Glucose transport is an essential metabolic event that is characteristic of all eukaryotic cells. With respect to skeletal muscle, contraction is the most potent physiologic stimulus for glucose transport, resulting in up to a 50-fold increase during maximal exercise in humans (Katz et al. 1986). Whereas the full sequence of reactions underlying the activation of glucose transport during exercise has not been elucidated, there is good evidence that the activation of AMP-activated protein kinase (AMPK) plays an integral role in this process (Hardie & Sakamoto 2006).
Reactive oxygen species (ROS) consist of the superoxide anion and several of its derivatives, including hydrogen peroxide (H2O2). Skeletal muscle produces ROS at a low rate in the basal state and markedly higher rates during exercise (Murrant & Reid, 2001). Considering the evidence that exogenous ROS stimulate glucose transport in skeletal muscle and that contraction stimulates ROS production, it would seem intuitive that endogenous ROS production would play an important role in contraction-mediated glucose transport. Indeed this hypothesis was recently made, following the observation that addition of exogenous H2O2 to isolated muscle preparations at rest resulted in the activation of glucose transport and AMPK (Toyoda et al. 2004).
Now, we have tested this hypothesis (Sandström et al. 2006). Fast-twitch, extensor digitorum longus (EDL) muscles were isolated from mice and incubated in a physiological salt solution. The muscles were then stimulated to perform repeated contractions and analyzed for glucose uptake. Repeated contractions increased glucose uptake almost 3-fold, as measured with the glucose analogue: 2-deoxyglucose (2-DG) (Fig. 1). If the muscles were exposed to a general antioxidant, N-acetylcysteine (NAC), the contraction-mediated glucose uptake was diminished by about 50%. This indicated that endogenous ROS production played a significant role in contraction-mediated glucose uptake. To assess whether the NAC effect was specific for contraction, additional modes of glucose uptake activation were investigated. However, NAC did not affect insulin or hypoxia-mediated glucose uptake (Sandström et al. 2006). This suggested that the ROS involvement in glucose uptake was specific for exercise and not other physiologic stimuli.
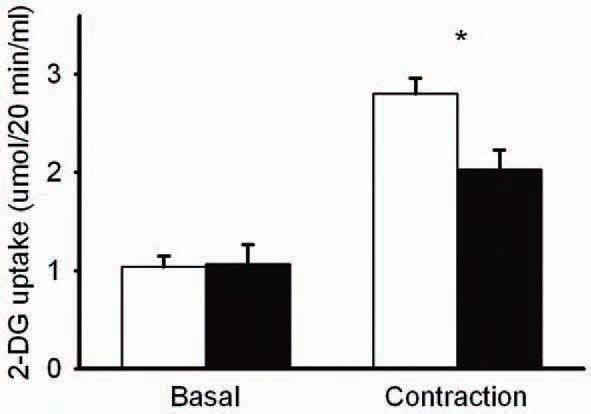
To establish that the NAC effect was associated with ROS metabolism, additional experiments were performed. We found that both intracellular ROS accumulation and alterations in glutathione status (reflects increases in ROS production) were blocked by NAC (Sandström et al. 2006). Thus NAC was functioning as an antioxidant.
To confirm the species of ROS involved in contraction-mediated glucose uptake, we performed two additional experiments. In one, we used another anti-oxidant, ebselen, which functions as a glutathione peroxidase mimetic that removes H2O2 in the presence of reduced glutathione (GSH). Ebselen also inhibited contractionmediated glucose uptake and to an extent similar to that seen with NAC (Sandström et al. 2006). This suggested that the ROS species involved in contraction-mediated glucose transport was H2O2.
We also studied muscles from mice that over-expressed manganese-dependent superoxide dismutase, which is the mitochondrial isoform of the enzyme that converts the superoxide anion to H2O2. Assuming that the production of superoxide anion is not altered compared with the wild type condition, this would result in an increased production of H2O2 and hence an increase in contraction-mediated glucose transport. Indeed, muscles from mice over-expressing the enzyme exhibited a glucose uptake rate following contraction that was about 25% higher than in wild type muscles (Sandström et al. 2006). Taken together, these results indicated that either H2O2, or one of its derivatives, was the ROS species involved in the activation of glucose transport during contraction. Based on our recent results, as well as other information available in the literature, we proposed the sequence of events depicted in Fig. 2 to explain how ROS stimulate glucose transport during exercise.
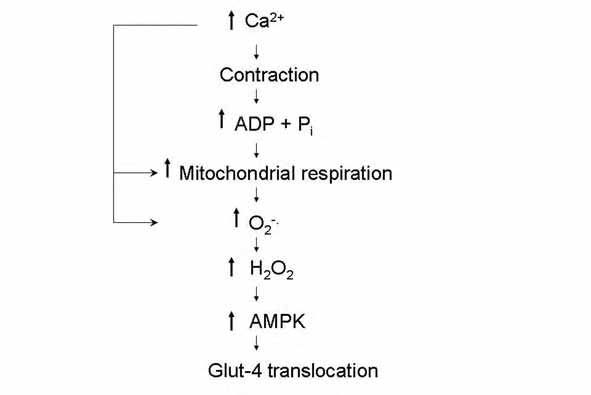
To study the mechanism whereby ROS mediate their effect on glucose transport, we measured the activity/phosphorylation state of AMPK. We found that NAC also inhibited the contraction-mediated increase in activity and phosphorylation (represents activation) of AMPK by about 50% (Sandström et al. 2006). Thus the ROS effect appeared to involve activation of AMPK.
Generally, ROS are associated with negative effects on body function, including muscle fatigue (Medved et al. 2004). However, recent studies have also implicated ROS as essential signaling molecules for normal physiological processes. The apparent paradox may be resolved by considering that excessive levels of, or prolonged exposure to ROS exert deleterious effects on cell function and viability, whereas low/physiological levels appear to be requisite for proper cell signaling and function. To this, one can add the possibility that different species of ROS affect various metabolic/structural targets to varying degrees depending on the conditions studied. A scheme to depict this view is given in Fig. 3. To illustrate the point one can imagine a situation where excessive amounts of ROS are produced during heavy exercise resulting in premature fatigue (i.e. a decrease in cell function). In this situation anti-oxidants would be expected to delay fatigue and hence have a positive effect on cell function, as has been demonstrated elsewhere
(Medved et al. 2004). However, in a situation where one relies heavily on glucose transport (eg, prolonged, submaximal exercise) and during which physiological amounts of ROS are produced, anti-oxidants could have deleterious effects.

So what can one conclude from the data? The conclusion is that, depending on the conditions, antioxidants can have either positive or negative effects on muscle performance.
References
Andrade FH, Reid MB, Allen DG & Westerblad H (1998). Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509, 565-575.
Hardie DG & Sakamoto K (2006). AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology 21, 48-60.
Katz A, Broberg S, Sahlin K & Wahren J (1986). Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol 251, E65-E70.
Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X & McKenna MJ (2004). N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 97, 1477-1485.
Murrant CL & Reid MB (2001). Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech 55, 236-248.
Sandström ME, Zhang SJ, Silva JP, Reid MB, Westerblad H, & Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in skeletal muscle. J Physiol 575, 251-262. 2006.
Toyoda T, Hayashi T, Miyamoto L, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Inoue G, Otaka A, Sato K, Fushiki T & Nakao K (2004). Possible involvement of the alpha1 isoform of 5’AMP-activated protein kinase in oxidative stress stimulated glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab 287, E166-E173.