
Physiology News Magazine
Reciprocal communication between L-type Ca2+ channel and ryanodine receptor in frog skeletal muscle
During skeletal muscle activation, L-Type Ca2+ channel opens the ryanodine receptor. In turn, the ryanodine receptor changes the properties of L-Type Ca2+ channel
Features
Reciprocal communication between L-type Ca2+ channel and ryanodine receptor in frog skeletal muscle
During skeletal muscle activation, L-Type Ca2+ channel opens the ryanodine receptor. In turn, the ryanodine receptor changes the properties of L-Type Ca2+ channel
Features
Roberta Squecco, Chiara Bencini, Claudia Piperio, & Fabio Francini
Department of Physiological Sciences, University of Florence, Firenze, Italy
https://doi.org/10.36866/pn.57.23

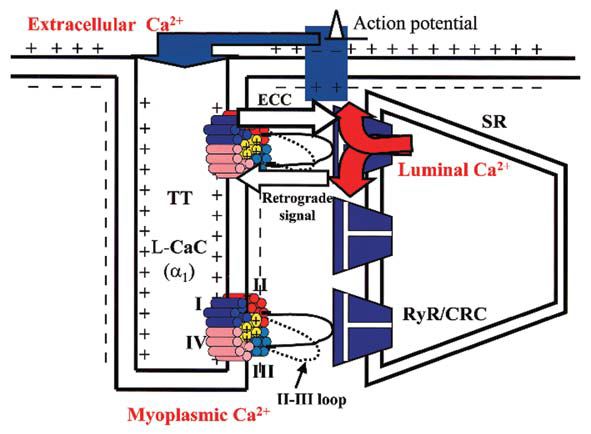
Depolarisation of the surface membrane in skeletal muscle fibres causes the excitation-contraction coupling (ECC) by triggering a series of events that ends with the contraction of the fibre. Depolarisation propagates as an action potential into the fibre via the T tubular system (TT) where the dihydropyridine receptors (DHPR) lie arranged in tetrads. Each unit of the tetrad is also a voltage operated L-type Ca2+ channel (L-CaC) (Fig. 1), consisting of five subunits: α1, α2, β, γ and δ. The pore-forming unit α1 has an aminoacidic sequence organized in four repeated domains (I, II, III, IV), each containing six transmembrane segments (S1-S6). The S4 segments contain positively charged aminoacid residues (yellow cylinders in Fig. 1). When the TT membrane is depolarised the charged S4-segment senses the new potential and moves. This movement, recorded by electrophysiological techniques, is called intramembrane charge movement (ICM). ICM determines the opening of the L-CaC and the movement of the loop between the II and III domain. Consequently, the coupled ryanodine receptor/Ca2+-release channel (RyR/CRC) of the sarcoplasmic reticulum (SR) opens and the luminal Ca2+, stored in the SR can flow into the myoplasm (Figs. 1 and 2).
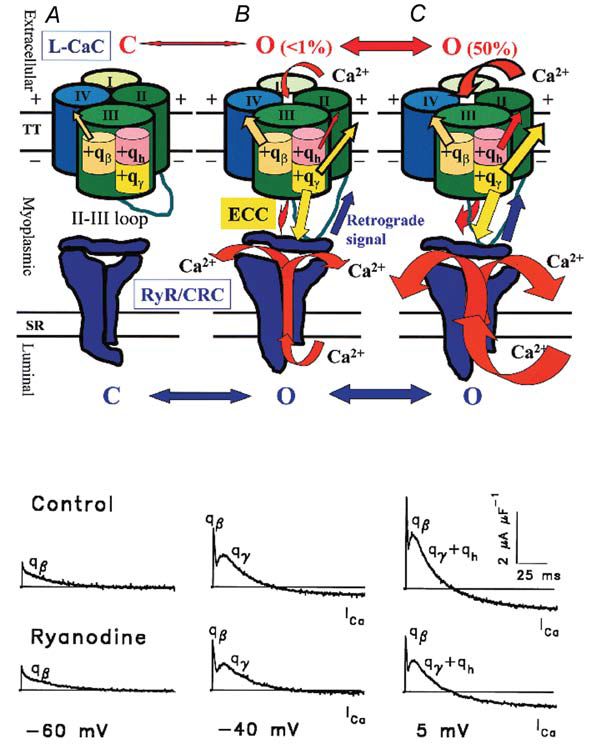
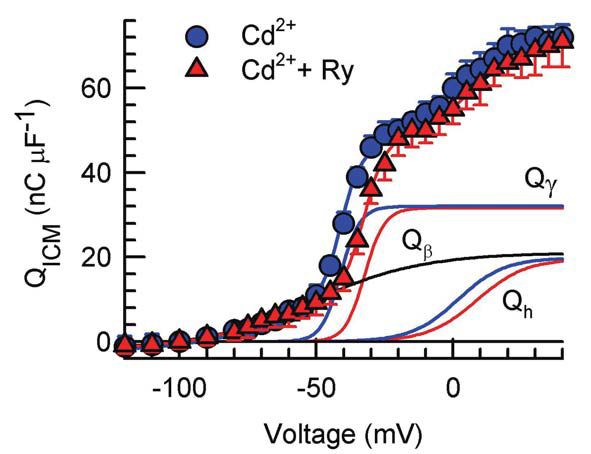
ICM involves three groups of charges with different voltage thresholds and voltage dependence. In fact, in normally polarized skeletal muscle fibres, ICM has been resolved into three components, an early qß, evoked by any depolarising step, and two delayed components, qγ and qh (Francini et al. 2001; Squecco et al. 2004) evoked only by voltage steps more positive than a certain threshold (about –56 and –38 mV in frog and rat, respectively). The time course of qß shows a monotonic decay (Fig. 2) and it seems not to be involved in ECC since the total amount of charge moved is not affected by pharmacological interventions directed at L-CaC such as Cd2+, nifedipine or alkanols. The hump–form charges qγ and qh can be resolved into two different ICM components by evaluating the voltage dependence of the amount of charge moved by depolarising steps above the voltage threshold (Fig. 3). In particular, the qγ movement triggers the opening of the coupled RyR/CRC, allowing a high Ca2+ flux from the SR into the myoplasm and promoting muscle contraction (Fig. 2B,C red arrow through RyR/CRC). The qh charge mobilization determines the opening of the sarcolemmal L-CaC that allows Ca2+ influx (ICa) from the external medium into the myoplasm (Fig. 2B,C red arrow through L-CaC). This may further enhance ECC by acting on RyR/CRC via Ca2+-induced Ca2+ release.
As described above, ECC is a one-way interaction between two proteins where the voltage-sensing promotes the RyR/CRC activation via mechanical and Ca2+-dependent coupling (Fig. 2B,C yellow and red arrows, respectively). Some evidence suggests a more complex functional communication. A reciprocal interaction, or cross-talk, is possible between these two proteins that involves specific regions of the coupled RyR/CRC. Thus, in addition to‘receiving’ the orthograde ECC signal from the L-CaC, RyR/CRC seems to ‘answer’ with a retrograde regulation (Fig. 2B,C, blue arrow) that increases the Ca2+ channel activity of the L-CaC (Nakai et al. 1998) causing an enhancement of ICa.
A point that needs clarification is a discrepancy in the literature whereby Csernoch et al. (1991) and García et al.(1991) report that similar manipulations to those used in Squecco et al. (2004) do not influence ICa, whereas Nakai et al. (1998) report that they do observe a retrograde regulation of ICa by the RyR. The idea to be considered is that manipulations of the RyR should reciprocally influence the behaviour of any charge components with which it makes allosteric contact (García et al. 1991; Csernoch et al. 1991; Jong et al. 1995; Huang, 1996; Chawla et al. 2002) and should spare only charge components that are not supposed to originate in the L-CaC and/or that are hardly involved in ECC.
In this debate, it appeared interesting to clarify the effects of the retrograde signal on ICa and which charge components are involved in the cross-talk between the L-CaC and RyR/CRC.
To this end, Squecco et al. (2004) did experiments in single fibres dissected from the semitendinosus muscle of the frog in voltage-clamp condition using the double-Vaseline-gap method (Francini et al. 2001). A number of pharmacological interventions were carried out that sought to interfere with a) L-CaC, by using channel blockers such as Cd2+ (Francini et al. 2001), nifedipine and 1-alkanols (heptanol and octanol), and b) RyR/CRC, by using specific blockers such as ryanodine (Garcia et al. 1991) and ruthenium red (RR) (Csernoch et al. 1991), known to suppress Ca2+ release from RyR/CRC.
We found that nifedipine reduced the amount of qγ and qh moved by ~90 % and ~55 % respectively, whereas 1-alkanols completely abolished them. Both interventions spared qβ. Ryanodine and RR did not affect the amount of qγ and qh moved, but shifted their voltage dependence (Fig. 3) and that of ICa activation more positively by ~4-9 mV (Fig. 4); conversely, Ryanodine and RR spared qβ. The effect of ryanodine together with those of nifedipine and 1-alkanols, demonstrate that qγ and qh both reside in the DHPR/L-CaC and represent separate independent processes from qβ. The parallel voltage dependence of qh and ICa is in agreement with the hypothesis that qh is the sensor for the L-CaC opening and that qγ is the sensor for ECC. Moreover, our results demonstrate that, in the absence of RyR/CRC blockers, the opening of the RyR/CRC facilitates qγ and qh movement as well as the opening of L-CaC by a retrograde signal.
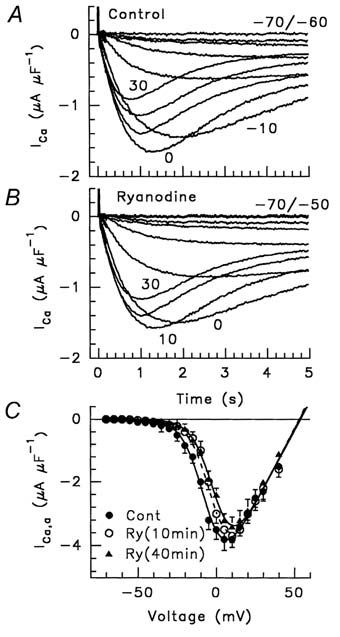
We thus confirm earlier reports that manipulations of the RyR/CRC reciprocally influence the qγ charge (Huang, 1996) and ICa (Nakai et al. 1998) and we report the novel finding that qh is influenced as well. Taken together, the above observations have deepened our understanding of the cross-talk hypothesis between the L-CaC and RyR/CRC, clearly suggesting that a kind of retrograde regulation by RyR/CRC facilitates the movement of the qγ and qh charge and the opening of L-CaC and, in turn, the ECC process.
References
Chawla S, Skepper JN & Huang CL-H (2002). Differential effects of sarcoplasmic reticular Ca2+-ATPase inhibition on charge movements calcium transients in intact amphibian skeletal muscle fibres. J Physiol 539, 869-882.
Csernoch L, Pizarro G, Uribe I, Rodrìguez M & Rìos E (1991). Interfering with calcium release suppresses Ιγ, the “hump” component of intramembranous charge movement in skeletal muscle. J Gen Physiol 97, 845-884.
Francini F, Bencini C, Piperio C & Squecco R (2001). Separation of charge movement components in mammalian skeletal muscle fibres. J Physiol 537, 45-56.
García J, Avila-Sakar AJ & Stefani E (1991). Differential effects of Ryanodine and tetracaine on charge movement and calcium transients in frog skeletal muscle. J Physiol 440, 403-417.
Huang CL-H (1996). Kinetic isoforms of intramembrane charge in intact amphibian striated muscle. J Physiol 107, 515-534.
Jong D-S, Pape PC & Chandler WK (1995). Effect of sarcoplasmic reticulum depletion on intramembranous charge movement in frog cut muscle fibers. J Gen Physiol 106, 659-704.
Nakai J, Sekiguchi N, Randos TA, Allen PD & Beam KG (1998). Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J Biol Chem 273, 13403-13406.
Squecco R, Bencini C, Piperio C & Francini F (2004). L-type Ca2+ channel and ryanodine receptor cross-talk in frog skeletal muscle. J Physiol 555, 137-152.
