
Physiology News Magazine
Regard the end
Harnessing physiology to provide better understanding of the mechanisms underpinning frailty
Features
Regard the end
Harnessing physiology to provide better understanding of the mechanisms underpinning frailty
Features
https://doi.org/10.36866/pn.123.20
Joseph Taylor, University of Nottingham, UK
Professor John Gladman, Nottingham University Hospitals NHS Trust and University of Nottingham, UK
Professor Paul Greenhaff, University of Nottingham, UK
Frailty is an increasingly used age-related concept, and we aim to highlight here why physiological research, particularly at a system level, can help address the global challenge posed by frailty. Life expectancy has been increasing at approximately 2 years per decade for the last 150 years, with those aged 65 years and over set to represent 1 in 4 of the UK population by 2039 (House of Lords report, 2013). Evidence from the UK Office for National Statistics (Office for National Statistics Statistical Bulletin, 2017) demonstrate that much of this increase in life expectancy since the second half of the 20th century has been due to increased survival of middle-aged people into old age.
For example, in 1950 a 65-year-old woman could expect another 15 years of life (for men this was 12 years); now she can expect another 28 years (for men it has risen to 24 years). Sadly, not all of this life extension is spent in good health. The average UK woman with a life expectancy of around 82 years can expect to live 19 of those years in poor health: men with a life expectancy of 79 years can expect to live 16 of those in poor health. What is needed is an increase in the “healthspan” rather than solely the lifespan.
“Frailty” is a term used in the scientific literature to refer to a state characterised by enhanced vulnerability to, and impaired recovery from, stressor events, when compared with a robust state. Examples of the consequences of the frailty state are the “geriatric conditions” of falls, confusion, incontinence, and immobility, which are common but not unique to frailty. These are the kinds of problems that do not kill older people but impair their quality of life. This makes frailty a potentially valuable target in attempts to improve the healthspan. Frailty, as we use the term here, is understood to indicate a state of loss of homeostatic reserve, due to the cumulative and interacting effects of impairments in organ structure and function primarily consequent to ageing processes. Although these same ageing processes can lead to states that are diagnosed as clinical conditions (e.g. arthritis, hypertension, kidney disease), frailty and multimorbidity are not synonyms. Similarly, although the ageing processes that lead to the loss of homeostatic reserve in frailty can eventually lead to reduced performance and hence the ability to perform everyday tasks, frailty and disability are also not synonyms, and it is possible to have lost reserves without reaching the point of producing disability.
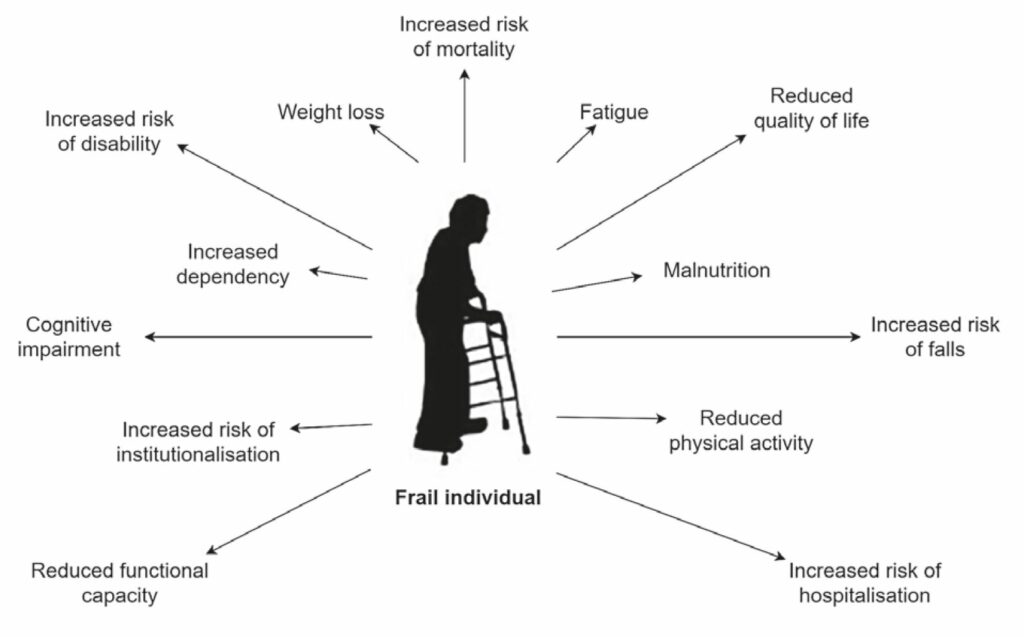
Moreover, not all old people are frail (the reported prevalence rises from 15% in 65 year olds to over 40% in 80 year olds (O’Caoimh et al., 2018, Song, Mitnitski and Rockwood, 2010)). Whilst we use the term to refer to an intrinsic biological state, we are aware that the word can be used in its lay sense, where it is often understood to have pejorative implications, such as pitiable or implying moral deficiency. With these caveats, Fig. 1 illustrates the traits associated with frailty.
As we have defined it, the conditions contributing to frailty are those identified as contributing to physiological decline during ageing, such as sarcopenia, inflammation, dementia, loss of appetite, etc. It is as yet unclear why these ageing processes develop at different rates in different people, or what comprises the cellular and molecular mechanisms directing the trajectory of age- related decline. Hence, why the frailty state is reached at an earlier stage in some people and delayed in others is unknown. In this article we posit that frailty (the reduction of which is the overall target of ageing research) is a multi-system concept, and therefore an integrative systems physiology perspective is likely to be the most illuminating and productive approach. The breadth of physiological systems affected by the ageing processes, and crucially how they interconnect to lead to dysfunction that manifests at the whole-body level is currently unknown.
There is limited but growing evidence that both ageing and the frailty state are not inevitable and unalterable processes, but are amenable to intervention. It seems likely that the differences in healthspan seen in different geographical populations and in the same populations over time are due to environmental and lifestyle factors. Apart from existing health hazards such as obesity, smoking and excessive alcohol consumption, the other obvious and known candidates associated with both increased longevity and greater healthspan are habitual physical activity levels, exercise and diet (energy content and composition). The mechanisms by which factors affect the underlying ageing processes in people are not yet known. Similarly, it is not yet known if the impacts of negative environmental and lifestyle factors on longevity and healthspan can be reversed, or whether greatest attention should be given to targeting interventions in pre-frail people (people who present with frailty traits, but less than frail individuals) to slow the decline into frailty.
These unknowns will be best addressed by longitudinal interventional study designs in which temporal change is quantified at multiple levels (molecular, cellular, tissue) in multiple physiological systems concurrently. Given physiology aims to understand the mechanisms of living, from the molecular basis of cell function to the integrated behaviour of the whole body, these unknowns represent a real opportunity for physiologists to positively impact on research and public health outcomes. In keeping with this, in 2019 The Physiological Society published the Growing Older, Better report, which highlighted the role of physiology in achieving this mission. More recently, the House of Lords Science and Technology Committee published its report Ageing: Science, Technology and Healthy Living, which focused on how approaches from science and technology could be used to increase health span, to mitigate some of the negative effects of ageing, and to support older people living in poor health (House of Lords Science and Technology Committee., 2021).
How is a frail person identified?
Assessing whether a person is frail remains a challenge. There are two main approaches at present. One approach is to classify people as robust, pre-frail or frail on the basis of five traits, which are assessed by simple functional tests such as handgrip strength and walking speed, and questionnaires concerning perceived unintentional weight loss, physical activity levels, and exhaustion in everyday life (Fried et al., 2001). This approach views frailty as a physiological syndrome. The other approach to identification of frailty views frailty as the cumulative effect of multiple deficits upon systems, and in this approach a “frailty index”, based on the proportion of possible deficits an individual has, is used to classify their frailty status (Mitnitski et al., 2001). Both approaches predict future clinical outcomes in populations. But neither approach is yet sophisticated enough to provide an assessment of the degree to which an individual is vulnerable and in what way. As yet, it is not possible to identify frailty on the basis of the consequences of multiple deficits due to ageing processes: a mechanistic understanding of underlying pathophysiological events is lacking, and frailty is clearly entirely operationally defined.
Why should a physiologist be interested in frailty?
One of the limitations of ageing research to date has been attributable to experimental approaches focusing on individual physiological systems – approaches that have served us well when dealing with single diseases and in the basic understanding of single organs. With respect to ageing, separate studies have highlighted dysregulation in brain (reduced brain volume, increased lesion volume, microstructural integrity deterioration; Tian et al., 2020), skeletal muscle (reduced muscle cross- sectional area, increased muscle fat fraction; Cesari et al., 2006; Lewsey et al., 2020), and cardiovascular (increased left ventricular mass, ejection fraction, arterial stiffness (Nadruz Jr et al., 2016) systems in the decline to frailty. However, longstanding evidence
demonstrates the physiological phenotype of frailty encompasses simultaneous dysregulation in multiple systems.
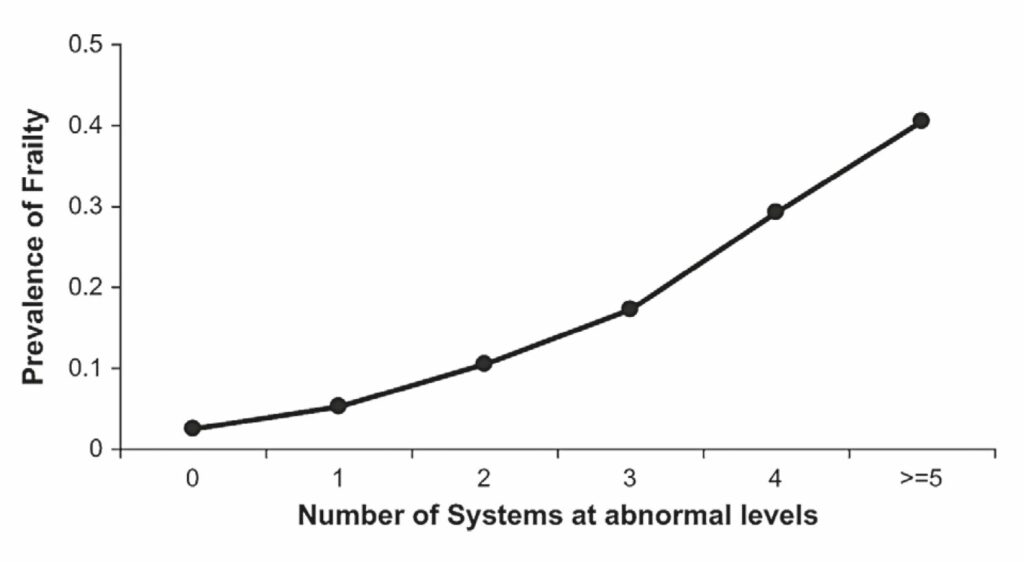
For example, Fried et al. (2009) demonstrated that the prevelance of frailty increased, from 2%-3% in people with no abnormal body systems to over 40% in those with five or more abnormal systems (Fig. 2), suggesting studies assessing individual physiological systems are providing limited insight into frailty. Promisingly, the multi-system dysregulation framework is now receiving more attention in the context of frailty (Ghachem et al., 2020; Li et al., 2015), and is bringing greater physiological
perspective to the condition. For example, analysis of purported biomarkers (anaemia, inflammation, IGF-1, dehydroepiandrosterone-sulphate, haemoglobin A1c, micronutrient status, adiposity and fine motor speed) of eight different physiological systems was performed in frail and non-frail elderly women. It was concluded that increased prevalence of abnormal biomarker status was related to the increased likelihood of frailty, such that abnormality in three or more biomarkers was deemed a significant predictor of frailty (Fig. 2).
Whilst effective in illustrating dysregulation in several suggested biomarkers during frailty, evidence from an amalgam of more robust physiological end-point measurements is still missing, particularly dynamic measurements of physiological function such as muscle protein synthesis, cardiac function, and cerebrovascular function. Accordingly, it will be important to build on these findings with more comprehensive structural and functional assessments of physiological function, in an integrated way, to uncover the main pathophysiological traits and mechanisms of frailty. Such an approach will inform the development of interventions to blunt or reverse the syndrome, which is likely to more efficacious than studying physiological systems in isolation.
How can collaboration help?
We assume that there are reversible mechanisms which could be enhanced or amplified to ultimately make a significant impact upon frailty and hence well-being in older age. These could be put in practice through novel drugs and custom-designed foods, and through individual changes to lifestyle and behaviours, and enhanced through economic and political processes. Key to making an impact on frailty is a requirement to harness research and technology that allows us to understand the mechanisms underpinning it and its progression. Collaboration between geriatricians and physiologists seems an obvious positive way forward, but also building collaboration between researchers in different physiological disciplines is a necessity, considering the multi-system nature of frailty described earlier.
Evidence from single-system studies suggests the amalgamation of brain, skeletal muscle, and cardiovascular research would be a productive starting point. Insight will be best served by dovetailing multi-modal approaches that allow complex physiological processes to be studied with granularity and relatively non-invasively in vivo in people, such as neuromuscular and motor function, anatomical and dynamic MRI, and stable isotope tracer measurements of metabolic turnover. This approach also lends itself well to the investigation of volunteers at multiple time-points in longitudinal study designs bringing detailed temporal insight of the trajectory of frailty and effectiveness of interventions to offset decline. Fortunately, we live in an age in which such technology platforms have been established, allowing investigation of multiple physiological systems to be undertaken concurrently in human volunteers, so we do not need to rely upon animal models of dubious validity.
Can physiology help to develop treatments for frailty?
Ultimately, research into degenerative syndromes should strive to aid in developing patient treatment options. In this regard, physiology will be fundamental in providing the foundations for frailty treatment, through identifying the syndrome’s pathophysiological characteristics, which can then be considered as targets for intervention. For example, it is widely accepted that frailty is concomitant with deterioration in skeletal muscle mass and metabolic resilience, but there is a scarcity of studies that have determined muscle mass and metabolism using gold standard approaches, such as MRI and stable isotope flux measurements. Further, studies have often been flawed by shortcomings in study design (e.g. an absence of gender stratification prior to analyses and/or the allocation of pre-frail and frail individuals into a single study group for analysis), reinforcing the need for clarification through physiological research. By effectively determining the relationships between frailty and skeletal muscle pathophysiology, interventions such as increased physical activity and/or resistance exercise could be further developed to help combat physiological dysregulation underpinning frailty. This of course begs the question of when is it best to intervene? Exercise intervention may not be at all feasible in the hyper-frail, but interventions that promote the mechanisms that are stimulated by exercise could be applied. An alternative would be to target the pre-frail using long-term and lifestyle interventions.
Where do we go from here?
Considerable research effort is required before the distinct pathophysiological processes and mechanisms of frailty development are comprehensively established. At present there is a lack of well-designed longitudinal studies investigating temporal relationships between frailty progression and wide-ranging and robust physiological end-points. Without longitudinal and multivalent data, it will be difficult to delineate mechanisms driving physiological decline into frailty. Furthermore, to date frailty research has primarily focussed on resting state measurements, which may not accurately reflect the dysregulation of dynamic homeostasis that some believe underpins frailty (Varadhan et al., 2008). In short, in the absence of acute infection, illness and injury, without the presence of external stressors such as feeding or physical activity, the dysregulation of physiological homeostasis in frailty may be subtle and undetectable. Accordingly, investigating frailty under conditions of controlled physiological stress was identified as a significant knowledge gap some time ago (Fried et al., 2005), and remains relatively unaddressed. This represents a real research and public health opportunity for physiologists.
References
Cesari M et al. (2006). Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. The American Journal of Clinical Nutrition 83, 1142- 1148. http://doi.org/10.1093/ajcn/83.5.1142
House of Lords, Select Committee on Public Service and Demographic Change. (2013). Ready for ageing? Report of Session 2012-13. House of Lords, London: The Stationery Office. 1-105. https://publications.parliament.uk/pa/ld201213/ldselect/ldpublic/140/140.pdf
House of Lords Science and Technology Committee. (2021) Ageing: Science, Technology and Healthy Living. https://publications.parliament.uk/pa/ld5801/ldselect/ldsctech/183/18304.htm
Fried LP et al. (2005). From bedside to bench: research agenda for frailty. Science of Aging Knowledge Environment 2005(31), pe24. https://doi.org/10.1126/sageke.2005.31.pe24
Fried LP et al. (2001). Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 56, M146-M157. https://doi.org/10.1093/gerona/56.3.m146
Fried LP et al. (2009). Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 64, 1049-1057. https://doi.org/10.1093/gerona/glp076
Ghachem A et al. (2020). Evidence from two cohorts for the frailty syndrome as an emergent state of parallel dysregulation in multiple physiological systems. Biogerontology 22(1), 63-79. https://doi.org/10.1007/s10522-020-09903-w
Lewsey SC et al. (2020). Exercise intolerance and rapid skeletal muscle energetic decline in human age- associated frailty. JCI Insight 5(20), e141246. http://doi.org/10.1172/jci.insight.141246
Li Q et al. (2015). Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell 14, 1103-1112. https://doi.org/10.1111/acel.12402
Mitnitski AB et al. (2001). Accumulation of deficits as a proxy measure of aging. The Scientific World Journal 1, 323-336. https://doi.org/10.1100/tsw.2001.58
Nadruz Jr W et al. (2016). Cardiovascular dysfunction and frailty among older adults in the community: the ARIC study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 72, 958-964. http://doi.org/10.1093/gerona/glw199
O’Caoimh R et al. (2018). Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: a systematic review and meta-analysis. Annali Dell’Istituto Superiore di Sanita 54, 226-239. https://doi.org/10.4415/ann_18_03_10
Office for National Statistics Statistical Bulletin (2017). Health state life expectancies, UK 2014-2016. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/healthstatelifeexpectanciesuk/2014to2016
Song X et al. (2010). Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. Journal of the American Geriatrics Society 58, 681-687. https://doi.org/10.1111/j.1532-5415.2010.02764.x
Tian Q et al. (2020). Microstructural neuroimaging of frailty in cognitively normal older adults. Frontiers in Medicine 7, 546344. http://doi.org/10.3389/fmed.2020.546344
Varadhan R et al. (2008). Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mechanisms of Ageing and Development 129, 666-670. https://doi.org/10.1016/j.mad.2008.09.013
