
Physiology News Magazine
Relax in a flash – shedding light on vascular smooth muscle photorelaxation
Recent research has shown that the ability of light to relax vascular smooth muscle is due to the photochemical release of NO from a rechargeable chemical ‘store’. Studies of the recharging mechanism suggest that light-sensitive sources of NO are also likely to be present in other cell types
Features
Relax in a flash – shedding light on vascular smooth muscle photorelaxation
Recent research has shown that the ability of light to relax vascular smooth muscle is due to the photochemical release of NO from a rechargeable chemical ‘store’. Studies of the recharging mechanism suggest that light-sensitive sources of NO are also likely to be present in other cell types
Features
Eric Flitney
School of Biology, Division of Cell & Molecular Biology University of St Andrews, Scotland (from March 2004: Cell & Molecular Biology, Feinberg Medical School of Northwestern University, Chicago, IL, USA)
https://doi.org/10.36866/pn.54.20

Vascular smooth muscle (VSM) relaxes when irradiated with either visible or ultra-violet (UV) light. This remarkable response, called ‘photorelaxation’, was first described by Robert Furchgott and his colleagues over 40 years ago
(Furchgott et al. 1962).
Some 20 years later, in their landmark paper, Furchgott & Zawadski (1980) showed that certain vasodilators act on blood vessels by stimulating the endothelial cell lining to release a highly-diffusible, labile substance that relaxes VSM cells in the vessel wall.
This potent ‘relaxing factor’ was later shown to be nitric oxide (NO) (Palmer et al. 1987), synthesised in endothelial cells from the amino acid L-arginine by an enzyme called nitric oxide synthase, or NOS. Inevitably, comparisons were drawn between endothelium-dependent relaxations and photorelaxation. Some striking similarities were found and so it was suggested that photorelaxation might be due to a light-activated relaxing factor with properties akin to NO, or perhaps even to NO itself.
This was an attractive hypothesis and a relatively straightforward one to test. In the event, however, confusing reports appeared in the literature, some of which did not sit comfortably with this idea. First, vessels from which the endothelium had been removed would relax when exposed to light. Second, photorelaxation was reportedly enhanced in vessels from hypertensive animals, whereas endothelium-dependent relaxations were attenuated. Third, and most damaging of all, photorelaxation was shown to increase after blocking the enzyme that synthesises NO. (The last turned out to be a red herring when it was found that the NOS inhibitors used in these studies could release significant amounts of NO when exposed to light!)
Our recent studies of photorelaxation (Flitney & Megson, 2003) used functionally-intact segments of rat tail artery that were perfused at a constant flow rate and irradiated from time to time with either visible or UV light. The perfusion pressure was recorded continuously and vessels from rats with normal blood pressures were compared to those from a strain of animal that spontaneously develops high blood pressures, widely used as a model for human hypertension.
These experiments showed that irradiating an artery with visible light produced a transient relaxation: an initial, rapid loss of pressure that reversed fully during the period (6 min) of illumination. The vessel was then no longer able to respond to a second, identical period of irradiation given immediately after the first. However, we found that its sensitivity to light returned spontaneously when it was allowed to remain for a time in the dark. The recovery process, called ‘repriming’, displayed an absolute requirement for both endothelium-derived NO and tissue thiols.
Based upon these observations, we concluded that photorelaxation is due to the release of NO from a photosensitive chemical ‘store’, most likely one of the class of compounds called S-nitrosothiols (Megson et al. 1995; 2000).
The responses we obtained using UV light turned out to be more complex than those seen with visible light. These consisted of an initial rapid dilation followed by a partial reversal only, resulting in a residual level of reduced pressure that persisted for as long as the vessel remained illuminated. The form of the UV response suggested to us that it might be a composite one, made up of two vasodilator responses: a brief phasic component, superimposed on a sustained component.
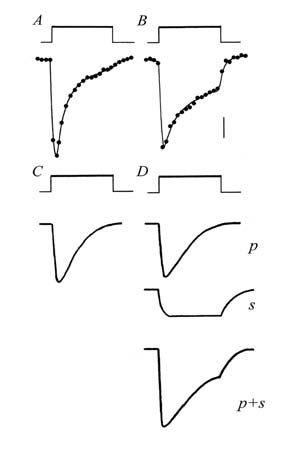
This idea, illustrated in Fig. 1, was confirmed when it was shown that the two components behaved quite differently. The phasic element was indistinguishable from responses obtained using visible light: it too showed refractoriness and the repriming process displayed an absolute requirement for endothelium-derived NO. By contrast, the sustained component did not show refractoriness and it was not dependent upon endothelium-derived NO. Other important differences emerged when vessels from normotensive rats were compared to those from spontaneously hypertensive animals. Thus, NO-mediated photorelaxation was attenuated in vessels from the hypertensive animals (Fig. 2), in line with similarly reduced endothelium-dependent relaxations, whereas the sustained component of the UV-induced response was enhanced.
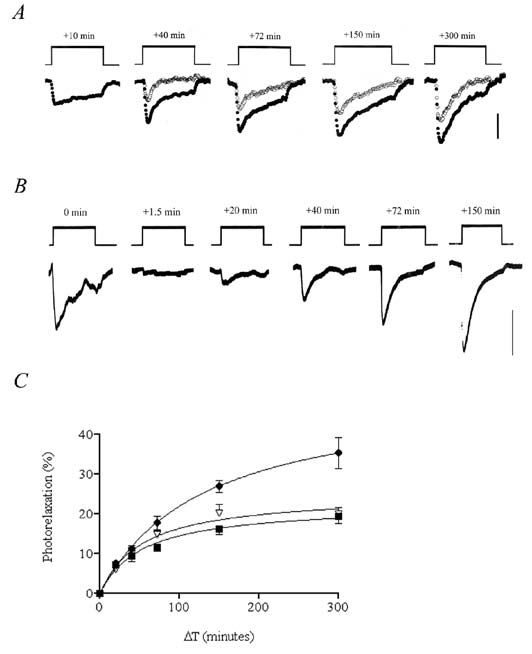
These latest findings establish the importance of NO in the mechanism of photorelaxation and of the endothelium as the source of NO needed to fuel the repriming process. They also highlight the fact that the response to light is greatly influenced by the prior history of a vessel. The duration of the interval between successive periods of illumination is clearly an important factor. The rate of flow of fluid through a vessel will probably prove to be important as well, since shear stresses associated with flow enhance NO synthesis by endothelial cells. Thus, the extent to which the store becomes replenished in the dark, and hence the size of the next in a series of responses to light, will largely depend upon these two variables.
Some important questions about photorelaxation remain unanswered. What is the physiological significance of a molecular store of NO in VSM? Is it a re-usable resource, one from which NO can be released during periods of increased demand, perhaps by way of some as yet unidentified ‘dark’ reaction(s); or is it simply the by-product of a ‘mopping-up’ process designed to chemically inactivate excess NO? Does the photosensitive character of the store have any physiological significance?
The answer to this is probably not, since relatively few blood vessels will see the light of day. It seems much more likely that this is an unavoidable consequence of the chemical nature of the store. Of course, this is not to say that it is of no interest or has no value to physiologists. Quite the reverse in fact, because the ability to release NO virtually instantaneously inside cells at will provides us with an important analytical tool, analogous to our use of synthetic ‘caged’ compounds to elevate other physiologically-active ligands inside cells.
So do studies of photorelaxation have wider implications for cell physiology? It seems so. There is no reason to suppose that VSM cells are unique in containing a photosensitive store of NO. Indeed, from what we know of the probable chemical nature of the store and of the repriming mechanism, we can be reasonably certain that similar stores do exist elsewhere. This opens up exciting possibilities for future studies of NO-mediated phenomena in other cell types.
References
Flitney FW & Megson, IL (2003). Nitric oxide and the mechanism of rat vascular smooth muscle photorelaxation. J Physiol 550, 819-828.
Furchgott RF, Ehrreich SJ & Greenblatt E (1961). The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol 44, 499-519.
Furchgott RF & Zawadski JV (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373-376.
Megson IL, Flitney FW, Bates J & Webster R (1995). Repriming of vascular smooth muscle photorelaxation is dependent upon endothelium-derived nitric oxide. Endothelium 3, 39-46.
Megson IL, Holmes SA, Magid KS, Pritchard RJ & Flitney FW (2000). Selective modifiers of glutathione biosynthesis and‘repriming’ of vascular smooth muscle photorelaxation. Br J Pharmacol 130, 1575-1580.
Palmer RMJ, Ferrige AG & Moncada S (1987). Nitric oxide release accounts for the biological activity of EDRF. Nature 327, 524-526.
