
Physiology News Magazine
Research at high altitude
The factors that regulate brain blood flow at high altitude are unclear. Our recent findings show that the balance of oxygen (O2) and carbon dioxide (CO2) pressures in arterial blood explains 40% of the change in brain blood flow upon arrival to high altitude (5050 m). We have also shown that blood vessels in the brain respond to changes in CO2 differently at high altitude compared to sea level – a factor that can influence breathing responses as well.
Features
Research at high altitude
The factors that regulate brain blood flow at high altitude are unclear. Our recent findings show that the balance of oxygen (O2) and carbon dioxide (CO2) pressures in arterial blood explains 40% of the change in brain blood flow upon arrival to high altitude (5050 m). We have also shown that blood vessels in the brain respond to changes in CO2 differently at high altitude compared to sea level – a factor that can influence breathing responses as well.
Features
Sam Lucas (1) and Philip Ainslie (2)
1: University of Otago, Dunedin, New Zealand
2: University of British Columbia Okanagan, Kelowna, BC, Canada
https://doi.org/10.36866/pn.84.37


Research at high altitude provides an excellent means to examine chronic and acute adaptation to hypobaric hypoxia. Humans native to high altitude provide an ideal cohort in which to study biological adaptation to the chronic environmental stress of living at high altitude. Given the time necessary to study any chronic adaptation as well as the profound limitations on quality of life and related expense, studying the effects of chronic hypoxia at sea level using hypobaric or hypoxic chambers is not feasible. The Pyramid Research Laboratory in Nepal (Fig. 1), located at an altitude of 5050 m above sea level, is an ideal in-the-field laboratory in which to conduct high-altitude experiments. A central focus for our work is on the influence that cerebral blood flow (CBF) has on breathing control, the changes that occur at high altitude and how this relates to the development of periodic breathing.

Overview
Since first reported by Severinghaus and colleagues (1966), increased CBF upon initial exposure to high altitude and subsequent normalisation after a period of acclimatisation has been well described; yet, the underlying mechanisms for these changes remain unclear. The myriad of factors involved (e.g. neuronal, biochemical and mechanical), the nature of their interaction, and their progressive changes with acclimatisation underscore the complexity of this process. The regulation of CBF during exposure to high altitude will, at least partly, depend on the degree of ventilatory sensitivity (i.e. hypoxic and hypercapnic ventilatory responses) and on the cerebrovascular responses to hypoxia and CO2.
Ventilatory control of CBF
The partial pressures of arterial carbon dioxide (PaCO2) and oxygen (PaO2) play a major role in CBF regulation. Upon arrival to high altitude, those individuals who exhibit a ‘brisk’ ventilatory response will have a higher PaO2 and lower PaCO2 than those individuals who have a ‘blunted’ ventilatory response. Thus, changes in CBF at high altitude may be related to the balance of PaO2/ PaCO2 – a balance determined by an individual’s ventilatory sensitivity to the hypoxic environment.
Cerebrovascular sensitivity
The degree of CBF responsiveness to acute changes in PaO2 and PaCO2 (i.e. cerebral reactivity) is another important factor that determines CBF. Experimental studies that have examined the influence of exposure to hypoxia on cerebral reactivity are limited and have variable results, while the changes that occur across acclimatisation have not been investigated to date.
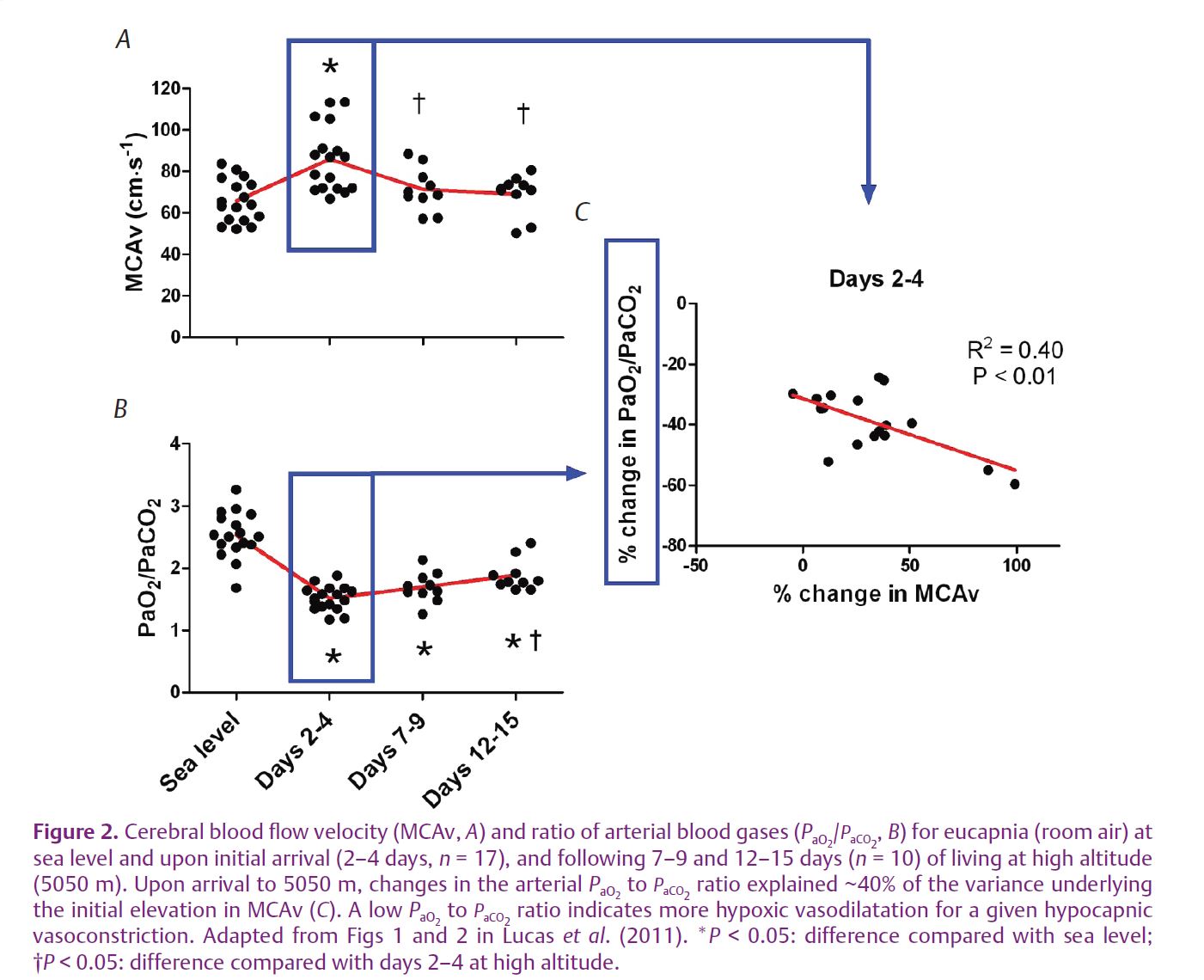
In a recent publication in The Journal of Physiology (Lucas et al. 2011), we report on the relation between changes in CBF and the balance of arterial blood gases upon ascent to high altitude and during a two week acclimatisation period to 5050 m. We observed that the changes in the balance of arterial blood gases explain approximately 40% of the variance underlying the initial elevation in CBF at high altitude (Fig. 2). Furthermore, our data indicated that, within 7–9 days of living at high altitude, normalisation of CBF occurs due to hypoxia-induced elevations in ventilation. This influence leads to a higher PaO2-to-PaCO2 ratio and therefore less hypoxia-induced dilatation and more hypocapnia-induced constriction in the cerebral circulation. The additional factors that account for the ‘other’ 60% are likely to include neuronal and local factors (e.g. endothelium-derived nitric oxide) as well as CBF changes that are driven by alterations in blood pressure, given the reported reductions in cerebral autoregulation at high altitude (Van Osta et al. 2005).
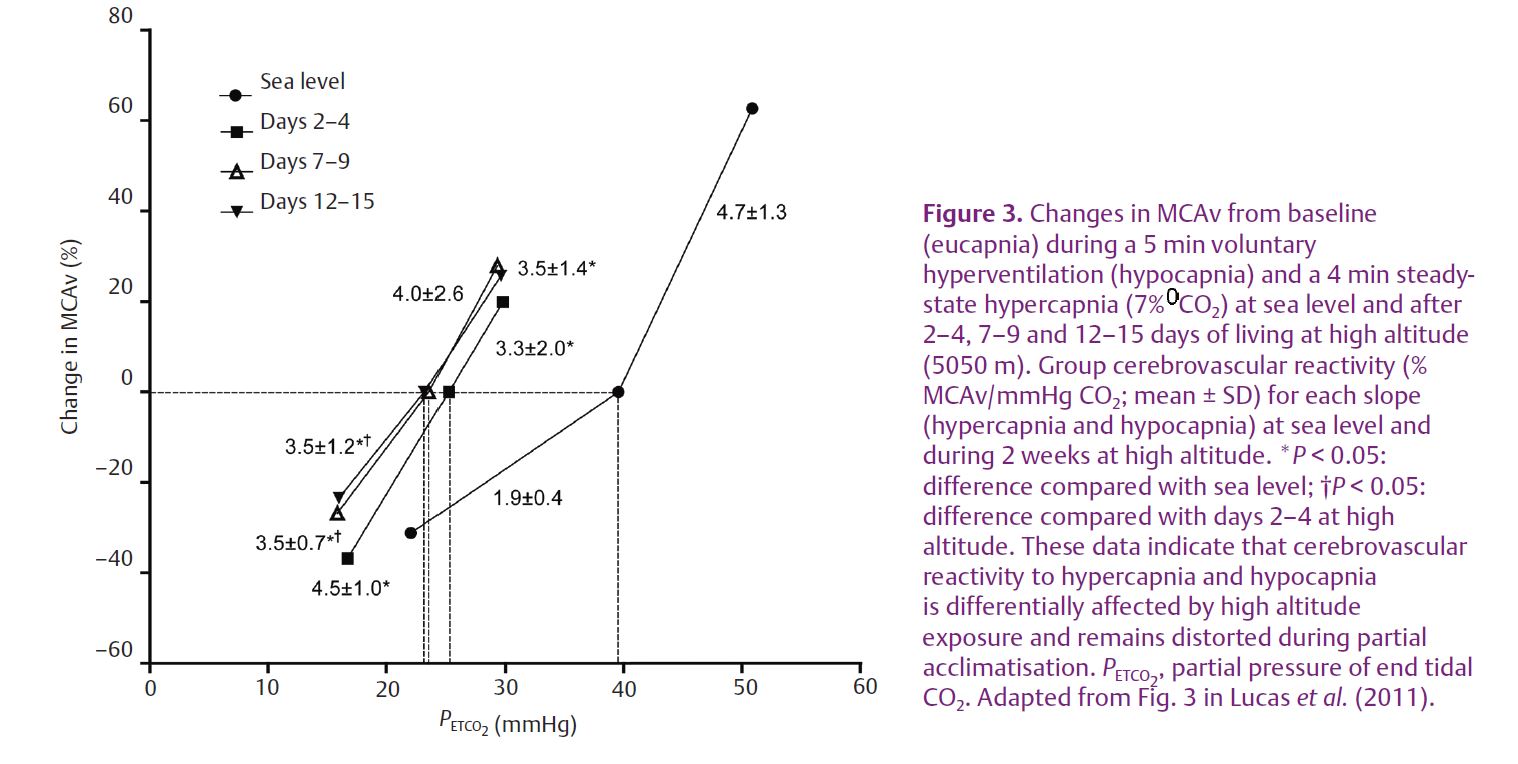
At sea level, steady-state cerebrovascular reactivity is more sensitive to increases in PCO2 (i.e. hypercapnic reactivity) than decreases (i.e. hypocapnic reactivity). Our high-altitude data indicate that this relation is reversed upon initial arrival to high altitude; notably, however, following partial acclimatisation, hyper- and hypocapnia cerebrovascular reactivity become similar to one another and both different from sea-level responses (Fig. 3).
Implications
The implications of alterations in CBF and cerebrovascular reactivity to CO2 are numerous, including a potential impact on breathing stability. For example, reductions in cerebrovascular CO2 reactivity develop a heightened ventilatory response to CO2 and an unstable breathing pattern in patients with congestive heart failure (e.g. Xie et al. 2005) and obstructive sleep apnoea (e.g. Burgess et al. 2010).
Tight control of the cerebrovascular CO2 reactivity provides an important protective reflex to minimise changes in brain [H+] at the level of the central chemoreceptor, and thereby stabilise breathing during fluctuating levels of chemical stimuli (Ainslie & Duffin, 2009). Consequently, a reduction in CBF hypercapnic responsiveness to CO2 and/or an augmentation in the hypocapnic range, as we observed in our recent study, may account in part for the development of periodic breathing commonly observed in newcomers to high altitude. The causative link between altered cerebrovascular function and the occurrence of breathing instability at high altitude is incompletely understood and thus requires further investigation.
Future directions
To date, research on CBF responses at high altitude has almost entirely focused on the effects during wakefulness. Extrapolation of these findings to the sleeping state, however, should be done with caution given the dynamic changes in CBF during sleep and the potential for differences in CBF responsiveness between wakefulness and sleep. We also know that the wakefulness drive to breathe is vital for maintaining rhythmical breathing, and in its absence (i.e. during sleep) levels of PaCO2 become critical in regulating the breathing pattern (Skatrud & Dempsey, 1983). Thus, given the link between breathing pattern and CBF, both of which are altered at high altitude when awake, the changes in breathing stability and CBF that occur during acclimatisation may be important in the development of periodic breathing at high altitude. Therefore, obtaining sleeping state measures of CBF responses at high altitude seems like a logical step for improving our understanding of the pathophysiology of periodic breathing, and has the potential to provide insight into why symptoms of altitude sickness are more prevalent after waking.
References
Ainslie PN & Duffin J (2009). Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement and interpretation. Am J Physiol Regul Integr Comp Physiol 296, R1473–R1495.
Burgess KR, Fan J-L, Peebles KC, Thomas KN, Lucas SJE, Lucas RAI, Dawson A, Swart M, Shepherd K & Ainslie PN (2010). Exacerbation of obstructive sleep apnea by oral indomethacin. Chest 137, 707–710.
Lucas SJE, Burgess KR, Thomas KN, Donnelly J, Peebles KC, Lucas RAI, Fan J-L, Basnyat R, Cotter JD & Ainslie PN (2011). Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050 m. J Physiol 589, 741–753. http://jp.physoc.org/content/589/3/741.long
Severinghaus JW, Chiodi H, Eger II EI, Brandstater B & Hornbein TF (1966). Cerebral blood flow in man at high altitude: role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res 19, 274–282.
Skatrud JB & Dempsey JA (1983). Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol 55, 813–822.
Van Osta A, Moraine J-J, Melot C, Mairbaurl H, Maggiorini M & Naeije R (2005). Effects of high altitude exposure on cerebral hemodynamics in normal subjects. Stroke 36, 557–560.
Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B & Russell D (2005). Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med 172, 371–378.
