
Physiology News Magazine
Role of cationic amino acid transporters in the regulation of nitric oxide synthesis in vascular cells
Anwar Baydoun and Giovanni Mann review the physiological and molecular characteristics of CAT transporters and discuss the role of L-arginine transport in regulating NO synthesis
Features
Role of cationic amino acid transporters in the regulation of nitric oxide synthesis in vascular cells
Anwar Baydoun and Giovanni Mann review the physiological and molecular characteristics of CAT transporters and discuss the role of L-arginine transport in regulating NO synthesis
Features
Anwar R. Baydoun
Biosciences Department, University of Hertfordshire
Giovanni E. Mann
Centre for Cardiovascular Biology & Medicine, King’s College London
https://doi.org/10.36866/pn.52.12

The discovery in 1987 that endothelium-derived relaxing factor is nitric oxide (NO) was followed a year later with reports that the cationic amino acid L–arginine is the physiological precursor for NO and in 1998 with the award of the Nobel Prize in Physiology and Medicine for the discovery of NO as a key signalling molecule. Several groups, including ours, have investigated the characteristics and regulation of cationic amino acid transporters(CAT-1, CAT-2A, CAT-2B, CAT-3) in vascular cells. In this article we review the physiological and molecular characteristics of CAT transporters mediating L–arginine influx and discuss the role of Larginine transport in regulating NO synthesis. As there are several excellent reviews on the transduction pathways associated with NO biosynthesis and signaling, we aim to provide only a brief overview.
L–arginine-nitric oxide pathways
Nitric oxide is a free radical with a multitude of physiological and pathophysiological functions. Highly labile NO is synthesised from the cationic amino acid L–arginine following oxidation of the terminal guanidino-nitrogen by a family of enzymes referred to as NO synthases (NOS). Three distinct isoforms, endothelial (eNOS or NOS I), neuronal (nNOS or NOS II) and inducible (iNOS or NOS III), have been identified and are known to be derived from separate genes and regulated by diverse signalling pathways (reviewed by Alderton et al. 2001).
Both eNOS and nNOS are constitutively expressed, dependent upon Ca2+ and calmodulin for activation, and generate picomolar amounts of NO over short periods of time following agonist stimulation. Under normal physiological conditions, generation of NO via the constitutive endothelial L–arginine-NO pathway appears to be a key regulator of vascular tone, maintaining the vasculature in a basal state of vasodilatation. nNOS on the other hand mediates diverse neuronal functions accounting, for instance, for the nitridergic component of peripheral nonadrenergic, non-cholinergic neurotransmission.
In contrast to its constitutive isoforms, synthesis of NO via the inducible pathway involves induction of a Ca2+/calmodulin-insensitive iNOS, previously identified in macrophages and now known to be induced in a wide variety of cell types including endothelial and smooth muscle cells. Expression of this enzyme is time-dependent, involves de novo protein synthesis and can be inhibited by protein synthesis inhibitors and glucocorticoids. Once induced the activity of the enzyme is sustained over prolonged periods and generates quantitatively more NO compared to its constitutive isoforms. Overproduction of NO by iNOS functions as an important mediator of inflammatory responses and has been implicated in the pathogenesis of various inflammatory and autoimmune disorders (reviewed by Hobbs et al. 1999).
Source of substrate for NO synthesis
The source of L–arginine for NO synthesis appears to depend on the physiological state and biosynthetic pathway being activated. L–arginine on it own does not significantly alter blood pressure in vivo, nor does L–arginine alter coronary perfusion pressure or the tension developed by isolated blood vessels in vitro. These findings suggest that basal NO synthesis is not limited by substrate availability. L–arginine is present in high concentrations in endothelial cells (~0.8 mM), and the Km of eNOS for L–arginine is <0.01 mM, with maximal stimulation occurring in the presence of 0.03-0.1 mM L–arginine. Thus availability of L–arginine may not be rate limiting for the relatively small quantities of NO released under basal conditions. The same appears to be true for transient stimulation of NO production by various agonists, including bradykinin and acetylcholine. However, when a stimulus is applied repeatedly over prolonged periods, endogenous substrate becomes rate limiting. Under these conditions availability and transport of exogenous L–arginine restores responses previously rendered tolerant by repeated or prolonged agonist administration. In this context, studies in cultured endothelial cells, perfused tissues, whole animals and man have shown that exogenous L–arginine can reverse inhibition of eNOS by inhibitor analogues of L–arginine itself, and enhance or sustain agonist-induced release of NO from the endothelium. Interestingly, the endotheliumdependent component of cyclic AMP-mediated relaxation in rat pulmonary arteries is critically dependent on availability of extracellular L–arginine (see Hucks et al. 2000).
The dependence of NO production on exogenous L–arginine is perhaps more apparent with iNOS, the high output system that generates nanomolar quantities of NO over prolonged periods. Release of NO by this enzyme is not only dependent on the presence of extracellular L–arginine, but also directly related to the rate of L–arginine transport.
These findings suggest that availability and transport of L–arginine can limit NO production under both physiological and pathophysiological conditions. There are, however, counter arguments, since vascular cells can metabolise L–citrulline via a truncated urea cycle to generate adequate amounts of L–arginine for sustained NO synthesis. Consistent with this hypothesis is that conversion of L–citrulline to L–arginine is enhanced in endothelial cells stimulated to release NO, as well as in macrophages and smooth muscle cells generating NO following activation with pro-inflammatory mediators. However, despite these increases in the generation of L–arginine from L–citrulline, our studies in intact cells have shown that in L–arginine deprived cells L–citrulline cannot sustain maximal rates of NO synthesis (Fig. 1). Moreover, as transport of L-citrulline into vascular cells is slow and 3-fold lower than rates measured for L–arginine, it seems unlikely that L–citrulline transport can sustain maximal rates of NO synthesis in vivo (Wileman et al. 2003)
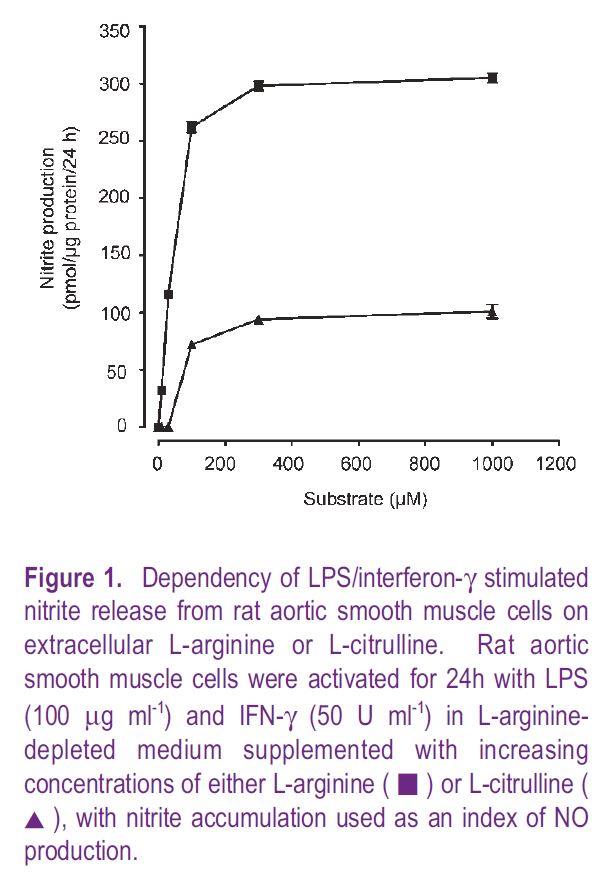
Modulation of L–arginine transport by nitric oxide
The critical role of exogenous L–arginine in regulating NO production begs the question of whether NO can modulate transport of L–arginine. Influx of L–arginine is increased transiently in endothelial cells stimulated with NO releasing agonists such as bradykinin, ATP or adenosine and elevated in cells expressing iNOS. Recent studies have shown that the NO donors (SNAP, dipropylenetriamine NONOate) acutely stimulate L–ARGININE influx, whereas longer exposure (1-4 h) to these NO donors inhibits transport. The inhibitory effects of prolonged NO exposure on L–arginine transport were attributed to the oxidation of sulfhydryl moieties within the transporter protein. At present there is limited evidence to suggest that L–arginine transport and NO synthesis are directly coupled. Instead, these two processes may be regulated independently since (i) increases in L–arginine transport occur despite inhibition of iNOS expression by dexamethasone, (ii) inhibition of iNOS with arginine analogues such as L-NAME does not alter cytokine-stimulated L–arginine transport in cultured cells and (iii) transfection of iNOS cDNA in cells which do not express iNOS either constitutively or in response to proinflammatory mediators (e.g. HEK293 cells) fails to cause significant changes in L–arginine transport despite a significant increase in NO production (unpublished observations). These findings argue against regulation of L–arginine transporter function by the activity of iNOS.
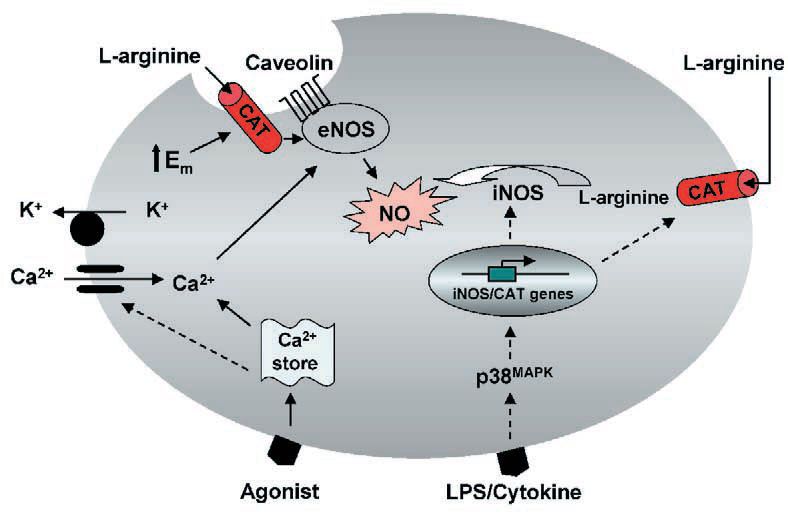
Based on the evidence available, it seems that enhanced L–arginine transport in iNOS expressing cells requires de novo protein synthesis. This may result from the activation by external stimuli of key kinase signalling cascades of which the p38 mitogen-activated kinase pathway may be critical (Baydoun et al. 1999). In contrast, transient increases in L–arginine transport observed in agonist-stimulated endothelial cells may be secondary to a membrane hyperpolarization induced by vasoactive agonists (Fig. 2). Nevertheless, we would still like to postulate that increased cationic amino acid transporter activity provides a mechanism for sustaining L–arginine supply during prolonged NO production.
Transport systems mediating influx of L–arginine
L–arginine is predominantly transported across cell membranes via specific sodium-independent transporter(s) selective for cationic amino acids. This pathway was originally termed system y+. Although other systems, including b+, bo,+, Bo,+ and y+L, have been identified as transporters for cationic amino acids, these carriers also accept a wider range of substrates, including neutral amino acids (Devés & Boyd, 1998). Thus, the only selective cationic transporter expressed in vascular cells still remains system y+.
System y+ was initially thought of in terms of a ‘one-protein-one transport activity’ paradigm. However, the evolution of recombinant DNA technology and the development of new kinetic experimental approaches have unveiled a more complex picture. It is now evident that transport of L–arginine, and indeed other cationic amino acids, involves several distinct proteins which are distinguishable by their structure, distribution and affinity for cationic amino acids.
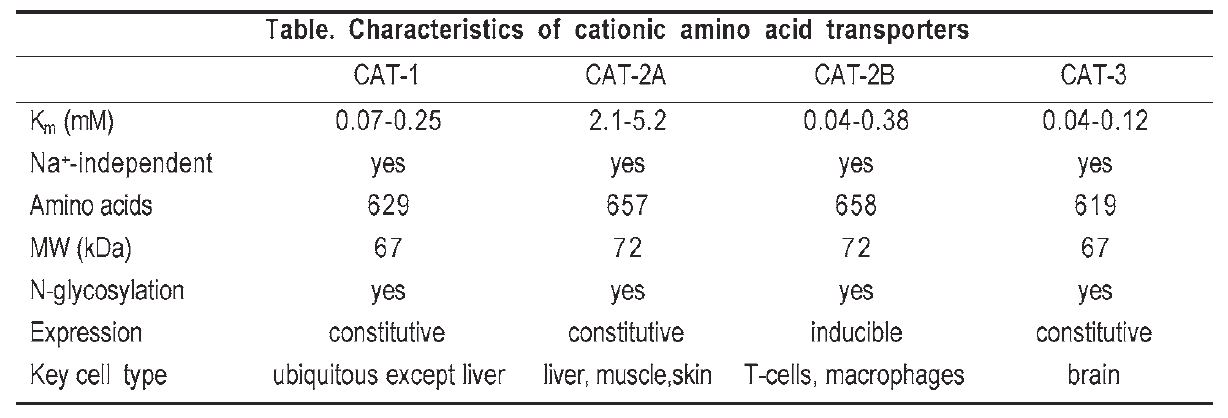
At least five different CAT proteins designated CAT-1, CAT-2A, CAT2B, CAT-3 and CAT-4 have been identified in different mammalian tissues. Some of the key characteristics of these proteins are discussed below and summarised in the Table. CAT-4 appears to lack cationic amino acid transport activity, and CAT-3 is expressed predominantly in brain and thymus tissue and also interacts with neutral amino acids.
Molecular identification, structure and function of CATs
Of the five proteins, CAT-1, a highaffinity electrogenic transporter, was the first to be characterised at a molecular level. This protein was cloned serendipitously by a group searching for the ecotropic murine leukemia virus receptor (ecoR), and shown to mediate cationic amino acid transport when expressed in Xenopus oocytes. The deduced amino acid sequence of the cloned cDNA revealed a 622 amino acid glycoprotein of about 67 kDa with 12 to 14 transmembrane spanning domains.
A truncated CAT-2 gene was cloned shortly after CAT-1 and initially named Tea (T-cell early activation receptor) because of its induction early in the response of normal T cells to mitogens. The full length cDNA which encodes a 658 amino acid protein (CAT-2B) was subsequently isolated and shown to have a 61 % homology with CAT-1 (see Table) and 98 % homology with CAT-2A. It is now known that CAT2A and CAT-2B are the products of differentially spliced mRNAs. The deduced sequence of these two proteins differ by only 20 amino acids within a stretch of 41 amino acids in an alternatively spliced region in the predicted fourth extracellular loop.
Unlike CAT-1 and CAT-2A, CAT2B is an inducible protein (at least in macrophages) with a high affinity for L–arginine (Km: 0.04 – 0.38 mM) comparable to that of CAT-1, despite its high sequence identity with CAT2A. This strongly indicates that the divergent domain in the predicted fourth loop of CAT-2 may be critical for substrate recognition and/or translocation. Indeed, substitution of this domain in CAT-2B with that of CAT-2A resulted in a chimeric protein that had a low affinity for L–arginine, while substituting this domain in CAT-2A with that of CAT-1 or CAT-2B resulted in a transporter with high affinity for L–arginine comparable to that of CAT-1 and CAT-2B. These variations in transport properties have recently been attributed to differences in two amino acid residues within the stretch of 42 amino acids in the alternatively spliced region of the human CAT isoforms (Habermeier et al. 2003).
At present we cannot distinguish between CAT-1 and CAT-2B in terms of their relative contribution to total L–arginine uptake under normal physiological conditions, nor can we specify which CAT directly supplies L–arginine to NOS in cell system. This latter point is important since there are reports that CAT-1, by virtue of its co-localisation with eNOS in membrane caveolae supplies substrate to this enzyme (Fig. 2), whereas CAT-2B, which is induced in parallel with iNOS, critically regulates supply of L–arginine to this enzyme. Moreover, the finding that iNOS mediated NO production is significantly reduced in peritoneal macrophages from CAT-2B-,- mice (Nicholson et al. 2001), strongly suggests a functional association between CAT-2B and iNOS, and a critical dependency of NO production on L–ARGININE delivery at least in peritoneal macrophages.
The notion that different NOS isoforms may be directly linked to distinct CAT(s) for substrate supply should however be viewed with caution, since this may be cell type dependent. Using isoform-specific probes in RNase protection analyses, we have identified transcripts for CAT-1, CAT-2A and CAT-2B in rat primary aortic smooth muscle cell cultures. Moreover, significant elevations in mRNA for all three CAT isoforms were detected following activation of cells with LPS and interferon-g (Baydoun et al. 1999). Enhanced transcript levels for CAT-1, -2A and -2B have also been observed in cardiac myocytes following treatment with IL-1b and IFN-g (Simmons et al. 1996). These findings have now lead to the realisation that transport of L–arginine may be more complex than the initial “one-protein-one transport activity” paradigm, and raise the question of the specific contribution of each CAT to total L–arginine transport in different cell systems. Specific knockouts and/or antisense strategies are clearly required to identify the key CAT(s) associated with the regulation of NO biosynthesis.
Conclusions
The identification of distinct transport proteins that mediate influx of L–arginine provides a basis for assessing the role of these transporters in regulating NO production, though questions remain concerning the signal transduction pathways involved in the coactivation of L–arginine transport and NO synthesis. Future understanding of the compartmentalisation of different CATs may permit the design specific probes that could be used to selectively manipulate expression and/or function of different CATs. This would undoubtedly allow for the physiological role of these proteins and their involvement in various diseases to be established. Indeed there are indications that altered L–arginine transport may contribute to the pathophysiology of cardiovascular disorders. Acute administration of L–arginine restores endothelium-dependent vasodilatation in hypercholesterolemic patients, and both acute and chronic administration of L–arginine also normalises impaired endotheliumdependent relaxation in vessels isolated from cholesterol-fed rabbits. Recent evidence indicates that pregnancy-associated diseases, such as gestational diabetes, intrauterine growth retardation and preeclampsia, can induce phenotypic changes in the fetal vasculature which result in alterations in the L–arginine-NO signalling pathway (see review by Mann et al. 2003). Advances in the molecular biology of cationic amino acid transporters and intracellular signalling pathways provide a basis for further investigating the regulation of L–arginine transport in NO generating cells in health and disease.
References
Alderton WK, Cooper CE & Knowles RG (2001). Nitric oxide synthases: structure, function and inhibition. Biochem J 357, 593615.
Baydoun AR, Wileman SM, Wheeler_Jones CP, Marber MS, Mann GE, Pearson JD & Closs EI (1999). Transmembrane signalling mechanisms regulating expression of cationic amino acid transporters and inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem J 344, 265-272.
Deves R & Boyd CA (1998). Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78, 487545.
Habermeier A, Wolf S, Martine U, Graf P & Closs EI (2003). Two amino acid residues determine the low substrate affinity of human cationic amino acid transporter-2A. J Biol Chem 278, 19492-19499.
Hobbs AJ, Higgs A & Moncada S (1999). Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol 39, 191-220.
Hucks D & Ward JP (2000). Critical dependence of the NO-mediated component of cyclic AMP-induced vasorelaxation on extracellular L–arginine in pulmonary arteries of the rat. Br J Pharmacol 130, 9971004.
Mann GE, Yudilevich DL & Sobrevia L. (2003). Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83, 183-252
Nicholson B, Manner CK, Kleeman J & MacLeod CL (2001). Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem 276, 15881-15885.
Simmons WW, Closs EI, Cunningham JM, Smith TW & Kelly RA (1996). Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. Regulation of L–arginine transport and NO production by CAT-1, CAT-2A, and CAT-2B. J Biol Chem 271, 11694-11702.
Wileman SM, Mann GE, Pearson JD, Baydoun AR (2003). Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS and interferon-γ. Br JPharmacol (in press).
