Physiology News Magazine
Role of villus microcirculation in intestinal absorption: coupling of epithelial with endothelial transport
Capillaries in jejunal villi can absorb glucose at rates several hundred times greater per gram of tissue than in brain or contracting skeletal muscles. These high rates are made possible by delivery of glucose in high concentration from epithelium to capillaries via paracellular diffusion and solvent drag combined with increases of villus capillary blood flow and surface area
Features
Role of villus microcirculation in intestinal absorption: coupling of epithelial with endothelial transport
Capillaries in jejunal villi can absorb glucose at rates several hundred times greater per gram of tissue than in brain or contracting skeletal muscles. These high rates are made possible by delivery of glucose in high concentration from epithelium to capillaries via paracellular diffusion and solvent drag combined with increases of villus capillary blood flow and surface area
Features
J R Pappenheimer (1) & C C Michel (2)
1: Department of Biology, Harvard University, Cambridge, MA, USA
2: Division of Biomedical Sciences, Imperial College, London, SW7 2AZ, UK.
https://doi.org/10.36866/pn.56.16

Investigations of nutrient absorption from the small intestine have focussed almost entirely on transport into and out of the epithelial cells. However, the transport pathways extend beyond the epithelial absorptive cells to villus capillaries so that, in addition to the apical and lateral epithelial cell membranes, transport continues through the epithelial basement membrane and the walls of the villus capillaries into flowing capillary blood. Previous investigators have assumed that the basement membrane and capillary walls offer little resistance to absorbed solutes, and that the concentration differences across them are negligible. Our recent analysis of glucose absorption shows that this assumption is far from correct (Pappenheimer & Michel, 2003). The capillaries present a barrier comparable with that of the epithelium, and increases in villus capillary blood flow and surface area during absorption are an important part of the absorptive mechanism.

A schematic diagram of the transport pathways is shown in Fig. 1. Glucose enters the epithelial cells with Na+ on the SGLT-1 transporter and leaves by facilitated diffusion through the lateral membranes on Glut-2. It is seldom appreciated that the nucleus, mitochondria, and other membranous organelles occlude most of the cross sectional area of the epithelial cells (Buschmann & Manke, 1981). Consequently, diffusion into the basal regions of the cell is hindered and the preferential pathway lies through the lateral membranes immediately below the tight junctions. Subsequent transport to the abluminal surfaces of the capillaries is by convection and diffusion through the post-junctional intercellular channels. Thus paracellular transport is the connecting link between epithelium and capillaries, and there is strong anatomical and electrophysiological evidence that the channels are widened during Na+-coupled transport of sugars and amino acids (Madara & Pappenheimer, 1987; Pappenheimer & Volpp, 1992).
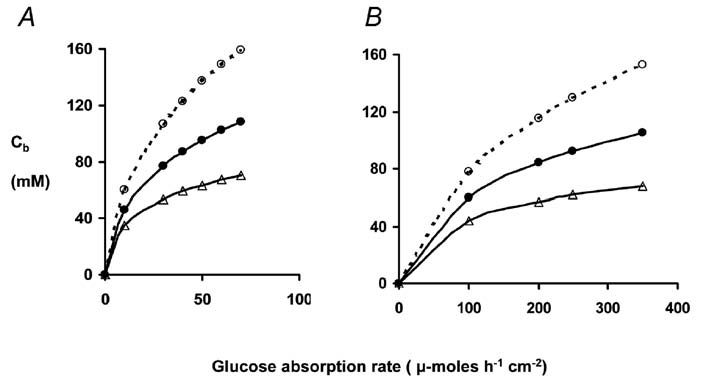
We have estimated glucose concentrations along the pathway as a function of glucose absorption rates in unanaesthetised rats and in normal human subjects. Only small concentration gradients are necessary to account for diffusion-convection in intercellular channels. However, large trans-endothelial concentration gradients are required to account for observed absorption rates of glucose into villus capillary blood. Figure 2 shows our estimates of glucose concentrations at the epithelial basement membrane immediately outside the capillaries as a function of glucose load and villus blood flow. With normal hyperaemic responses to glucose absorption the concentrations in abluminal fluid required for diffusion into capillary blood are in the range 60 -100 mM; without the normal hyperaemic response, concentrations exceeding 400 mM would be required to sustain observed absorption rates.
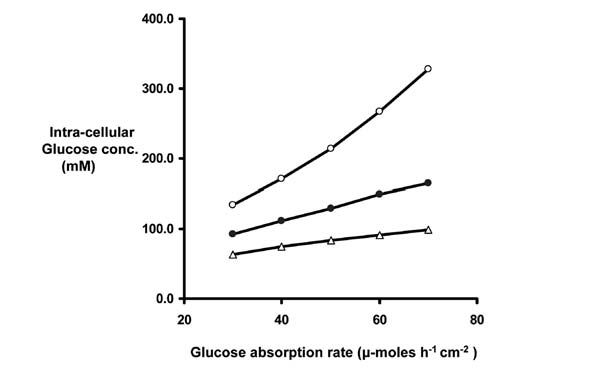
In addition to the large concentrations required for transport across villus capillaries, there may be significant gradients across the lateral membranes of epithelial absorptive cells. Calculation of these gradients, using available kinetic parameters of Glut-2 (Kellett, 2001), has led to estimates of intracellular glucose concentrations as shown in Fig. 3. From this it is easy to see how failure of blood flow to increase during absorption could lead to cellular glucose concentrations that compromise the efficiency of SLGT-1 upon which all glucose absorption depends.
Conclusions
The jejunal epithelium of normal animals can present the villus capillaries with absorptive loads that are several hundred times greater (per gram tissue) than found in capillaries elsewhere, including contracting skeletal muscle and brain. Far from being negligible, the villus capillary walls account for 50% or more of the resistance to glucose transport between the apical cytosol of the epithelial cells and the capillary blood during normal rates of absorption. Increases in villus capillary blood flow and permeabilitysurface area product are thus essential for sustaining high rates of glucose absorption. Na+-coupled concentrative transport of sugars and amino acids must trigger these increases in blood flow and permeability-surface area product; otherwise, concentrations in the sub-junctional fluids and within the epithelial cells would rise to levels that would compromise carrier mediated transport. Signals that trigger the microcirculatory responses remain to be discovered.
The low rates of absorption found in anaesthetized animals (Ugolev, 1987) may be attributed to inhibition of the normal microcirculatory responses associated with Na+-coupled transport by the epithelium. In anaesthetised animals with low villus blood flows, glucose concentrations outside the capillaries may exceed 150 mM (Fig. 2) and with increased gradients across the lateral membranes (Fig. 3) the intracellular concentrations will rise to 250300 mM. Total osmolarity, including the Na+ brought in with glucose, would then exceed 600 m-Osmolar, a value comparable to that found experimentally by Hällback et al. (1991).
August Krogh (1922) appears to have been the first to recognize the special problems posed by nutrient transport within the villi after absorption by the epithelium. The hypothesis outlined briefly in this essay provides a solution to these problems in terms of villus capillary permeability, surface area and blood flow on the one hand, coupled with properties of the epithelium on the other.
References
Buschman RJ & Manke DJ (1981). Morphometric analysis of the membranes and organelles of the small intestinal enterocytes. I Fasted hamster. J Ultrastruct Res 76, 1-14.
Hallbäck DA, Jodal M, Mannischeff M, & Lundgren O (1991). Tissue osmolality in intestinal villi of four mammals in vivo and in vitro. Acta Physiol Scand 143, 271-277.
Kellett JL (2001). The facilitated component of intestinal glucose absorption. J Physiol 531, 585-595.
Krogh A (1922). The Anatomy and Physiology of Capillaries. pp 244-249. Yale University Press, New Haven.
Madara JL & Pappenheimer JR (1987). The structural basis for physiological regulation of paracellular pathways in intestinal epithelium. J Memb Biol 100, 149-164.
Pappenheimer JR & Michel CC (2003). Role of the villus microcirculation in intestinal absorption of glucose: coupling of epithelial with endothelial transport. J Physiol 553, 561-574.
Pappenheimer JR & Volpp K (1992). Transmucosal impedance of small intestine: correlation with transport of sugars and amino acids. Am J Physiol 263, C480-C493.
Ugolev AM (1987). Membrane transport and hydrolytic enzymes under physiological vs acute experimental conditions. News in Physiol Sci 2, 186-190.