
Physiology News Magazine
Sensory vestibular information and vertebrate motor behaviour
Strains of mutant mammals where the inner ear degenerates at birth can be used to study the effect of the lack of sensory vestibular input during development. Recent data show the maturation of the membrane properties of central vestibular neurons is delayed, but not impaired, by the absence of sensory vestibular information. However, the motor behaviour of adult animals remains strongly affected
Features
Sensory vestibular information and vertebrate motor behaviour
Strains of mutant mammals where the inner ear degenerates at birth can be used to study the effect of the lack of sensory vestibular input during development. Recent data show the maturation of the membrane properties of central vestibular neurons is delayed, but not impaired, by the absence of sensory vestibular information. However, the motor behaviour of adult animals remains strongly affected
Features
Daniel Eugène, Nicolas Vibert, & Pierre-Paul Vidal
Laboratoire de Neurobiologie des Réseaux Sensorimoteurs, Université Paris Descartes – CNRS, Paris, France
https://doi.org/10.36866/pn.71.17

The central vestibular neurones located within the brainstem receive most of the sensory input coming from the vestibular sensory organs of the inner ear labyrinth, which detect head motion. These neurones play a major role in the processing of vestibular, visual and proprioceptive information related to body motion. They transform this sensory information into motor commands used to stabilize gaze and posture. Central vestibular neurones include several groups of neurones that have distinct functional roles. For instance, the neurones of the medial vestibular nucleus (MVN) are mainly involved in gaze and posture stabilization within the horizontal plane, i.e. they control the left-right orientation of the eyes, head and front part of the body.
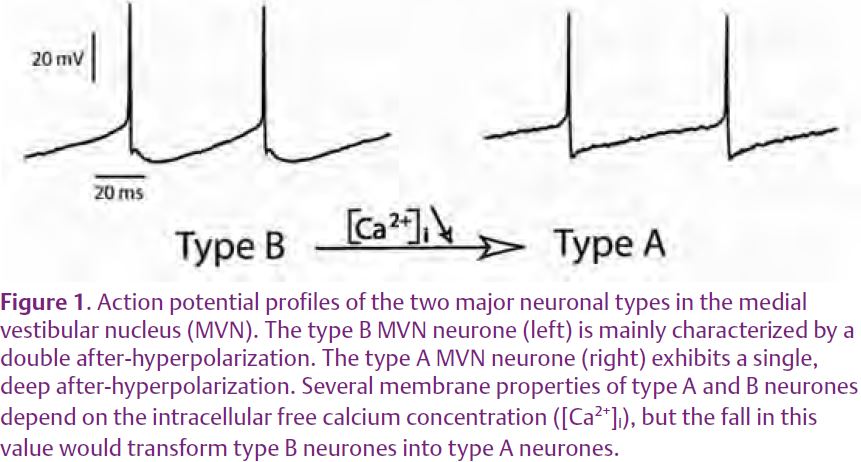
Over recent years, the neurones of the MVN, and to a lesser extent other vestibular neurones, have been studied both in vivo and in vitro in several mammalian species (Straka et al. 2005). Within each functional group, the membrane properties of individual vestibular neurones would shape their sensitivity and response dynamics. For instance, in vitro studies indicated that the MVN contains two major subtypes of neurones (type A and type B neurones), which differ in their spike and after-hyperpolarization shapes, as well as in the profile of their repolarization during inter-spike intervals (Fig. 1). This distinction has important consequences for their response dynamics (Straka et al. 2005). The presence of different subtypes of vestibular neurones that display distinct membrane properties and response dynamics seems to be a ubiquitous feature, since the vestibular neurones in chick and frog also subdivide into populations with different electrophysiological properties (Straka et al. 2005).
Studies in chick, mouse and rat have shown that central vestibular neurones are electrophysiologically immature at birth, and undergo a gradual process of post-natal maturation of their membrane properties. They are therefore a good model to study to what extent during development the activity of sensory afferents is necessary for the maturation of the neurones that receive and process sensory information in the central nervous system. The question is whether the signal carried by sensory vestibular neurones, which gives information on the head movement of the pups, is necessary for the maturation of the membrane properties of central vestibular neurons.
To address that question, we (Eugène et al. 2007) used a strain of vestibular-deficient mutant mice known as the KCNE1-/- mutant mice (Vetter et al. 1996). In these knockout mice, the KCNE1 potassium channel gene is inactivated, which leads to a selective degeneration of all sensory cells of the inner ear just after birth, while sparing the central nervous system (Warth & Barhanin, 2002). As a result, the KCNE1-/- mutant mice are deaf, their vestibular organs cannot encode head movements and the sensory vestibular afferents do not transmit any input. However, the central vestibular neurons look intact.
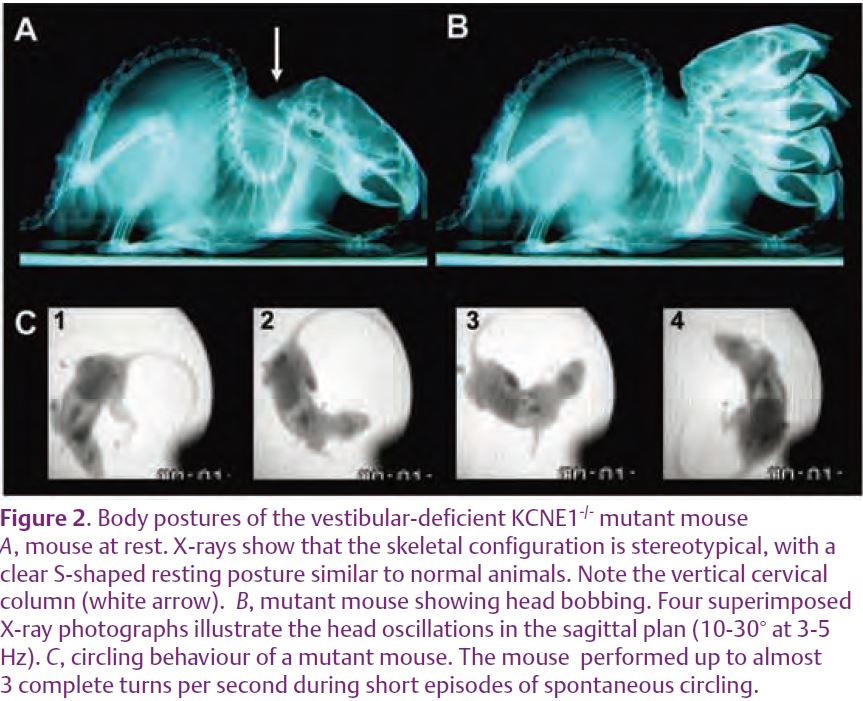
In adult normal animals, bilateral suppression of sensory vestibular input by destruction of the two inner ear labyrinths does not modify the resting posture, but these animals show a quasi-constant head bobbing behaviour (de Waele et al. 1989). The KCNE1-/- mutant mice display similar deficits (Fig. 2B), but show in addition a permanent “waltzer” phenotype (Vidal et al. 2004), i.e. their locomotion is frequently interrupted by episodes of rapid circling in a preferred direction (Fig. 2C). This suggests that suppression of sensory vestibular input induces the circling behaviour, but only when happening during a ‘sensitive period’ around birth. These mutant mice offer a unique opportunity to examine how central vestibular neurons mature in the absence of sensory input from the vestibular sensors, and to determine whether modifications of these neurones are responsible for this behaviour.
In juvenile KCNE1-/- mutant mice, the mutation resulted in a strong decrease in the expression of the calcium-binding proteins calbindin, calretinin and parvalbumin (which regulate the intracellular free calcium (Ca2+) concentration of neurones) within the MVN (Eugène et al. 2007). This decrease was associated with modifications of the membrane properties of MVN neurones, which provoked an 80%increase of the spontaneous discharge rate of type B neurones. These modifications were most likely related to an increased intracellular free Ca2+ concentration. Indeed, decreasing the intracellular free Ca2+ concentration within the recording pipette resulted in a decrease of the spontaneous discharge rate of type B MVN neurones back to control levels. In control mice with normal inner ear hair cells and no behavioural symptoms, a low Ca2+ concentration provoked an increase in the relative proportion of type A compared to type B neurones ( Fig. 1).
In adult mice, in contrast, there was almost no difference between the membrane properties of MVN neurons in KCNE1-/- mutant versus control mice. The expression levels of calbindin and calretinin were still lower in adult homozygous mutant animals than in normal adult mice, but the amount of these proteins expressed in MVN was much closer to normal than in juvenile mice.
Altogether, these data demonstrate that suppression of sensory vestibular inputs during a ‘sensitive period’around birth induces the circling/waltzing behaviour that characterizes numerous strains of mutant mammals, but that this behaviour is not due to persistent abnormalities of the membrane properties of central vestibular neurons. Indeed, maturation of the membrane properties of central vestibular neurons is delayed, but not impaired by the absence of sensory vestibular information, which suggests that the membrane properties of these neurons are at least in part genetically coded. Our data suggest that modulation of the expression levels of calcium-binding proteins in central vestibular neurons could play a major functional role. First, it could be involved in the recovery of the resting discharge of central vestibular neurons that follows an acute deafferentation of these cells in adult animals by infectious or traumatic lesions of the labyrinth, i.e. in the so-called vestibular compensation process (see Straka et al. 2005). Second, regulation of the intracellular free Ca2+ concentration might be used to modify the relative proportions of type A and type B MVN neurons according to functional demands.
References
De Waele C, Graf W, Josset P & Vidal PP (1989). A radiological analysis of the postural syndromes following hemilabyrinthectomy and selective canal and otolith lesions in the guinea pig. Exp Brain Res 77, 166-182.
Eugène D, Deforges S, Guimont F, Idoux E, Vidal PP, Moore LE & Vibert N (2007). Developmental regulation of the membrane properties of central vestibular neurons by sensory vestibular information in the mouse. J Physiol 583, 923-943.
Straka H, Vibert N, Vidal PP, Moore LE & Dutia MB (2005). Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76, 349-392.
Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF & Barhanin J (1996). Inner ear defects induced by null mutation of the isk gene. Neuron 17, 1251-1264.
Vidal PP, Degallaix L, Josset P, Gasc JP & Cullen KE (2004). Postural and locomotor control in normal and vestibularly deficient mice. J Physiol 559, 625-638.
Warth R & Barhanin J (2002). The multifaceted phenotype of the knockout mouse for the KCNE1 potassium channel gene. Am J Physiol Regul Integr Comp Physiol 282, R639-648.
