
Physiology News Magazine
Sex in studies
The first level of personalisation
Features
Sex in studies
The first level of personalisation
Features
Natasha A Karp, AstraZeneca, Cambridge, UK
Rohit Katial, AstraZeneca, DE, USA
Karen Thacker, AstraZeneca, Cambridge, UK
https://doi.org/10.36866/pn.118.32
Precision medicine is touted as the future; the approach to improve therapeutic outcomes by putting our patient at the centre of the healthcare system. Our sex is a fundamental evolutionarily conserved feature that divides our population into two. Yet our bench-to-bedside research neglects sex differences and assumes that knowledge gained from studying males will translate. Sex differences in disease prevalence, symptoms and progression, together with differences in treatment efficacy and side effects, are well accepted (Yoon, et al., 2014). Examples are being published that suggest the evidence base of medicine may be fundamentally flawed. We need to strive for the inclusion of both sexes and then explore the data assessing for a sex difference to ensure our research is representative of the target population. Embracing this change should improve translation and efficiency of science.



Current state of play
Twenty-seven years ago, the NIH Revitalisation Act mandated the inclusion of women in clinical trials. However, women remain under-represented and are rarely included in the early phases of clinical trials (Feldman et al., 2019). Exclusions are more likely in early testing with phase 1 and 2a trials more affected than phase 2b or 3. This risks drugs that might be more effective or safer in women being dropped from early clinical development due to poor efficacy or safety concerns in males.
In non-clinical research, researchers have highlighted that we predominantly study only one sex, typically males (Beery and Zucker, 2011, Fig. 1) and this bias has not changed in over 20 years (Mazure and Jones, 2015). The bias is apparent, even when the disease of interest is a female-prevalent disorder (Yoon et al., 2014).
The risk of studying only one sex has been highlighted recently in the study of pain. Scientists at McGill University in Montreal, Canada decided to not follow the convention of their community and instead studied both sexes. They concluded that the reaction to pain was highly dependent on the sex of the animal and whilst the symptoms of pain might look similar the pathway mediating the pain could be highly distinct (Sorge et al., 2015). Regulators, funders and publishers are pushing, across all stages of the research pipeline, to study both sexes. Whilst some progress is being made in the clinical research arena, non-clinical research is lagging behind. Most non-clinical studies, whether in vivo or in vitro, are conducted on only one sex, typically male. Whilst in clinical trials, inclusion of women is poor and analysis frequently fails to consider sex as a potential variable.
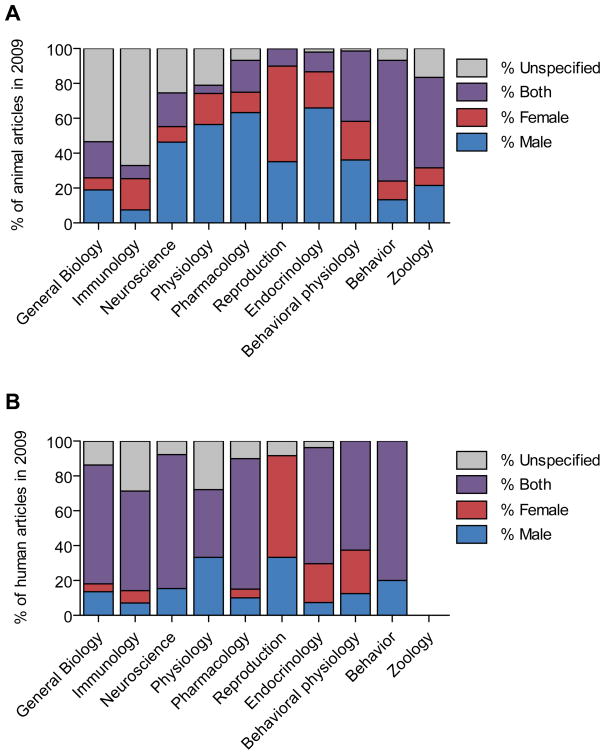
A paradigm shift through regulators, funders and journals forcing change
The historic focus on male subjects in clinical studies arose from risk management. In the 1970s, the priority was preventing adverse events on unborn foetuses, who had not consented to the clinical trial, and effectively banned the inclusion of women in clinical trials (Liu and Dipietro Mager, 2016). This was understandable in the wake of thalidomide where ten thousand children were born with malformations, at a time when oral contraceptives were new, their reliability uncertain and potential drug–drug interactions (DDIs) poorly understood. This position was reversed in 1993 with the NIH Revitalisation Act. Since then, regulators have instituted various policies to ensure proper inclusion of women in clinical trials. For example, the FDA has issued over 19 guidance documents and reports on the various aspects of inclusion of women in clinical research. Originally, women were only included once all relevant safety testing was complete. Now, the prerequisite non-clinical data requirements increase with the duration of the study and number of women included. It is often possible to enrol women in the very earliest single-dose studies with no more information than is required for men. The commonly used terminology of ‘first time in man’ may be misleading drug developers to believe that women should not be included until safety in men has been established. In reality, women can be included from the earliest clinical trials, using effective contraception (Clinical Trial Facilitation Group, 2014), when knowledge of the mechanism of action of the agent and the type of pharmaceutical agent are understood and the clinical trial meets criteria such as limiting time period of exposure and limiting the number of women exposed (ICH Harmonised Tripartite Guideline, 2009).
In pre-clinical research, multiple funding agencies, journals, and professional bodies have initiated programmes to encourage us to include both sexes in our research. As these advisory statements and initiatives had little impact, funders (such as the National Institutes of Health or Swedish Research Council) took the unusual step to mandate change and require consideration of sex in all applications from 2016. Likewise, journals such as the British Journal of Pharmacology recommend both sexes are studied and if not, a rationale must be included in the manuscript as to why it is not relevant to the experimental question (Docherty et al., 2019).
Why do we often study one sex?
The scientific method uses experiments to simplify a complex world to generate a testing space where we can isolate cause and effect. We then generalise the results. The level of complexity in biology is such that we have to make hard choices when we design experiments to generate “doable problems” allowing us to incrementally unravel the biological story. Historically, we have assumed sex isn’t a big deal, but this is now being questioned.
For in vitro research, the predominant reason for only studying one sex appears to be cultural; where most scientists perceive the sex of the cells is irrelevant (Shah et al., 2013). This position arises if the differences between the sexes is believed to arise from hormonal exposure. Recent research highlighting large scale differences in gene expression between the sexes has led to the theory that the underlying evolution for sex differences often works at the level of gene expression (Mealey, 2000). This is supported by the observation that sex differences exist between cells before the onset of hormonal exposure (Shah et al., 2013). Failure to consider sex in our experiments could lead to missed opportunities and erroneous conclusions. Consider the following example: researchers looking at the potential of muscle-derived stem cells to efficiently regenerate skeletal muscle in mouse models for muscular dystrophy found significant heterogeneity in the response (Deasy, 2007). Instead of walking away from the research, they explored the heterogeneity and found that the cell sex is a variable that considerably influences the regeneration abilities of the stem cells.
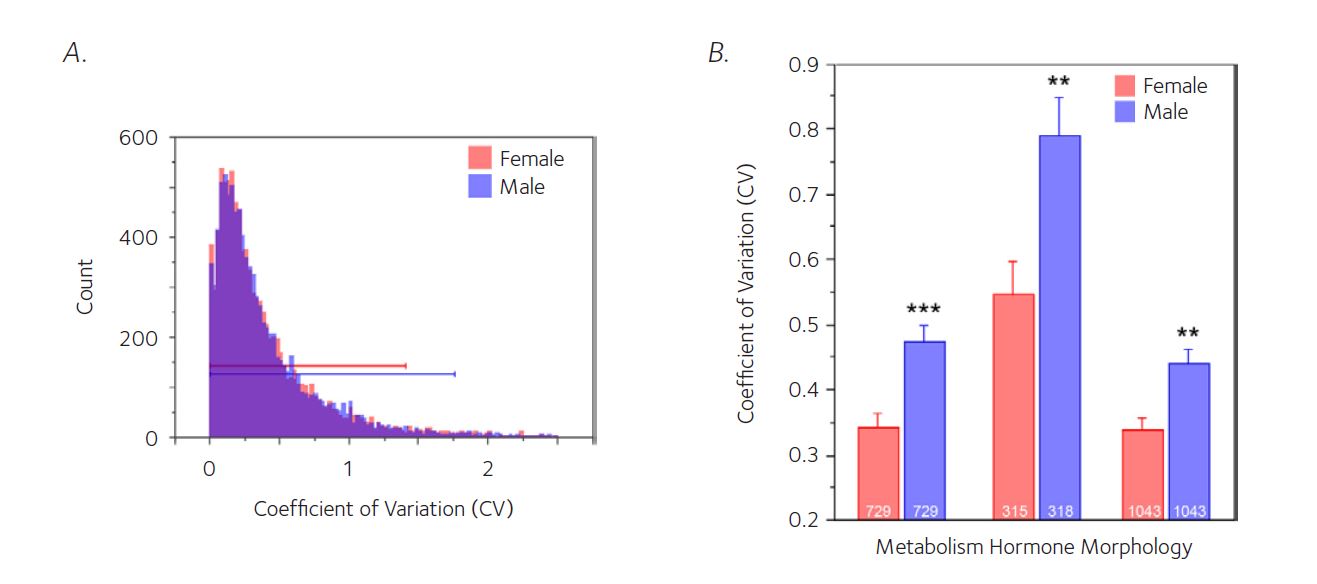
For in vivo research, the 3R (Replace, Reduce and Refine) ethical framework, has also encouraged the study of one sex. Historically, the Reduce element has been interpreted as a requirement to focus on minimising the absolute number of animals within a single experiment and thus encouraging us to study in a narrow testing space and to generalise the results. This approach ignores the fact that the production of male animals involves the production of female animals and there is a welfare burden from this under-utilised outcome from breeding. Males were then selected because it was believed that the oestrogen cycle resulted in female animals being more variable and consequently a higher number of animals would be needed within an experiment to detect the same size effect. A meta-analysis has highlighted this is a flawed belief, but the legacy remains (Prendergast et al., 2014, Fig. 2).
Practically, can we study both sexes?
In in vitro research, including sex as a biological variable can be challenging. Firstly, the sex is often not known for established cell lines as the chromosomes can become unstable. Secondly, many of these studies use immortalised lines generated from one individual. We cannot determine whether the differences would be due to a sex effect or individual differences. The critical step here is to understand the limitation of these studies and report the sex of cells when known. The increasing use of primary cells will allow studies to encompass sex as a biological variable going forward.
In in vivo research, there is resistance from misconceptions and practical challenges that hinder the use of both sexes. The most common argument against the use of both sexes is the misconception that you would need to double the sample size. In fact, it is recommended that you maintain your original design but split the animals for a treatment between the two sexes (McCarthy, 2015). The sensitivity is maintained because you now have a factorial design and the treatment effect is estimated from both sexes simultaneously (Karp and Reavey, 2019). This approach does mean these experiments are not powered to assess whether the treatment effect depends on sex, but will allow us to see a large difference if it exists. Studying both sexes does introduce additional practical challenges to ensure the process avoids the introduction of systematic bias and the experiments appropriately manage the welfare needs of the animals. For example, exposing a rodent to scents from unfamiliar animals or the opposite sex can lead to anxiety in the animal or changes in the experiment measure (Hurst, 2005). Different labs approach this challenge in different ways, some through cleaning whilst others accept the complex scent environment. We need to share our experiences and the strategies we develop so that we can learn how to manage this new paradigm.
In clinical trials, in spite of the requirement to include women, women are under-represented and frequently omitted from trials. Despite the change in regulator position, advancement of reliable contraceptives, improved DDI evaluation (Stewart et al., 2016) and greater knowledge of drug targets in embryo-foetal development, this stance has been slow to change (Liu and Dipietro Mager, 2016). The question should be not whether we should include female subjects but why shouldn’t we. To enable this, we need to ensure the criteria for inclusion of women in the first-in-human clinical trials are widely understood and risk assessments are based on literature and early non-clinical data when possible. Many phase I studies have a duration of less than 14 days and therefore can include small numbers of women of childbearing potential without any additional testing. Most phase II trials can include women by conducting preliminary embryo-foetal development studies earlier in drug development programmes (ICH Harmonised Tripartite Guideline, 2009). These steps combined with an inclusive approach to clinical study design will result in programmes that will better serve their patient demographic. In addition, these changes will increase the pool of volunteers/patients eligible for inclusion in clinical trials, which will speed up recruitment. This will help bring safe and effective medicines to the market more quickly, which is in the best interests of both patients and drug developers.
Driving change
Studying both sexes is essential and will improve experimental efficiency and enable social equality where our research pipeline represents the intended community. For over 20 years this issue has been discussed; however, sex bias is embedded in our research pipelines and culture. We don’t always report the sex, we often only collect data on one sex and we frequently ignore sex in the analysis when we have collected both sexes (Karp and Reavey, 2018). Change is hard. The tide appears to be turning; we are moving from recommendations to requirements. Within each of our domains, we need to provide the leadership to build coalitions that build a vision and support change. These coalitions should then consult to identify and find solutions to the blockers that are resisting the change and implement strategies to strengthen the driving forces for change (Karp and Reavey, 2018). The issues are multifaceted and the solutions to support this change need to be bespoke for your community. The question is, are you going to start this journey and embrace sex as a biological variable?
Natasha Karp, Rohit Katial and Karen Thacker are employees of AstraZeneca and own shares in the company. We have no conflicts of interest with the subject matter or materials discussed in this manuscript to declare.
References
Beery AK, Zucker I (2011). Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 35, 565 – 572. DOI: 565-72.10.1016/j. neubiorev.2010.07.002
Clinical Trial Facilitation Group (2014). Recommendations related to contraception and pregnancy testing in clinical trials. [Online] Available at: www.hma.eu/fileadmin/dateien/Human_ Medicines/01-About_HMA/Working_Groups/ CTFG/2014_09_HMA_CTFG_Contraception.pdf [Accessed 13 March 2020]
Deasy BM et al. (2007). A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. The Journal of Cell Biology 177, 73 – 86. DOI: 10.1083/jcb.200612094
Docherty JR et al. (2019). Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. British Journal of Pharmacology 176, 4081 – 4086. DOI: 10.1111/bph.14761
Feldman S et al. (2019). Quantifying sex bias in clinical studies at scale with automated data extraction. JAMA Network 2, e196700. DOI: 10.1001/ jamanetworkopen.2019.6700
Hurst J (2005). Making sense of scents: reducing aggression and uncontrolled variation in laboratory mice. [Online] National Centre for the Replacement, Refinement and Reduction of Animals in Research: London, UK, 1 – 8. Available online at: www.nc3rs.org.uk/sites/default/files/ documents/NC3RsarticleJaneHurst%20making%20 sense%20of%20scents.pdf [Accessed 13 March 2020]
ICH Harmonised Tripartite Guideline (2009). Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals M3 (R2). [Online] International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Available at: database.ich. org/sites/default/files/M3_R2__Guideline.pdf [Accessed 13 March 2020]
Karp NA, Reavey N (2019). Sex bias in preclinical research and an exploration of how to change the status quo. British Journal of Pharmacology 176(21), 4107 – 4018. DOI: 10.1111/bph.14539
Liu KA, Dipietro Mager NA (2016). Women’s involvement in clinical trials: historical perspective and future implications. Pharmacy Practice (Granada) 14, 708. DOI: 10.18549/PharmPract.2016.01.708
Mazure CM, Jones DP (2015). Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Women’s Health 15, 94. DOI: 10.1186/s12905-015-0251-9
Mccarthy MM (2015). Incorporating sex as a variable in preclinical neuropsychiatric research. Schizophrenia bulletin 41, 1016 – 1020. DOI: 10.1093/schbul/sbv077
Mealey L (2000). Sex differences: Developmental and evolutionary strategies. San Diego (California): Academic Press. DOI: 10.1016/S1090- 5138(01)00064-2
Prendergast BJ et al. (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 40, 1 – 5. DOI: 10.1016/j.neubiorev.2014.01.001
Shah K et al. (2013). Do you know the sex of your cells? American Journal of Physiology-Cell Physiology 306, C3 – C18. DOI: 10.1152/ajpcell.00281.2013
Sorge RE et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience 18, 1081 – 1083. DOI: 10.1038/nn.4053
Stewart J et al. (2016). Birth control in clinical trials: industry survey of current use practices, governance, and monitoring. Therapeutic Innovation & Regulatory Science 50, 155-168. DOI: 10.1177/2168479015608415
Yoon DY et al. (2014). Sex bias exists in basic science and translational surgical research. Surgery 156, 508 – 516. DOI: 10.1016/j.surg.2014.07.001
