
Physiology News Magazine
So what does cause the breakpoint of breath-holding?
The precise mechanism causing the breakpoint of breath-holding is unknown, but it is possible that breath-holding eventually stimulates diaphragm chemoreceptors that in turn cause the irresistible urge to breathe
Features
So what does cause the breakpoint of breath-holding?
The precise mechanism causing the breakpoint of breath-holding is unknown, but it is possible that breath-holding eventually stimulates diaphragm chemoreceptors that in turn cause the irresistible urge to breathe
Features
Michael J Parkes
School of Sport & Exercise Sciences, University of Birmingham, UK
https://doi.org/10.36866/pn.73.25

It is not normally possible for humans to breath-hold voluntarily until they lose consciousness (Parkes, 2006). This indicates that the voluntary act of breath-holding is terminated by some involuntary breakpoint mechanism. The nature of this involuntary breakpoint mechanism might be revealed by the three well known manoeuvres that prolong breath-hold duration:
- increasing lung inflation;
- breathing hyperoxic gas mixtures;
- lowering arterial PCO2 by previously hyperventilating.
These three suggest that the breakpoint mechanism might involve pulmonary stretch receptors and/or the carotid arterial chemoreceptors (not aortic chemoreceptors as these have no effect on breathing in humans). This in turn predicts that humans should be able to breath-hold indefinitively (until consciousness is lost) after pulmonary or carotid chemoreceptor denervation. Such denervation however has almost no effect on breath-hold duration (see Harty et al. 1996; Flume et al. 1996; Gross et al. 1976, in Parkes, 2006). So pulmonary stretch and carotid chemoreceptors are not obviously involved (although any proposed mechanism must still explain such prolongations).
There are three other, and less well known, manoeuvres that also prolong breath-hold duration and that might also reveal the breakpoint mechanism. The first appears to be voluntarily relaxing the diaphragm at the end of the breath-hold. Fowler (1954, in Parkes, 2006) made one of the earliest observations consistent with this (subsequently confirmed by Flume et al. 1994, in Parkes, 2006), by showing that inhaling an asphyxiating gas mixture at breakpoint enables a second (or even third) breath-hold, despite blood gas levels becoming progressively worse. This enabling effect is independent of the number of inhalations of asphyxiating gas, occurs even with a single inhalation with no net change in lung volume, or even apparently if inspiration is only attempted against a closed glottis (Rigg et al. 1974, in Parkes, 2006). Their one common feature might be in momentarily relaxing the diaphragm before using it to attempt to inspire again.
This in turn suggests that the‘holding’ of breath-holding might be achieved by continuously contracting the diaphragm slightly (Parkes, 2006), with the purpose of opposing the recoil from the chest.(It may be easier to oppose this recoil by slightly activating a big inspiratory muscle like the diaphragm rather than by intensely activating a small group of muscles such those keeping the glottis closed. Substantial diaphragm proprioreception is also unlikely as humans have little conscious sensation of the diaphragm).
During breath-holding such an unusual, prolonged diaphragm contraction may eventually result in under perfusion of the diaphragm, causing a stimulation of diaphragm muscle chemoreceptors that eventually generates the irresistible urge to breathe, i.e. the breakpoint of breath-holding. Stopping breath-holding by relaxing the diaphragm may then allow it to be reperfused, ending the stimulation of diaphragm chemoreceptors by metabolites. This hypothesis could explain the prolongation of breath-hold duration by increased lung inflation, hyperoxia and hypocapnia, with each delaying the onset of such diaphragm chemoreceptor stimulation. Even if diaphragm chemoreceptor stimula-tion is only perceived vaguely as discomfort, the ability to tolerate such discomfort will have a strong subjective component that could also explain the notorious variations in breath-hold duration seen both between and within subjects (Parkes, 2006).
This key role of the diaphragm in the breakpoint mechanism (Parkes, 2006) is supported by the second less well known breath-hold prolonging manoeuvre, paralysis (curarisation) of the diaphragm. In 1966–1969 Campbell et al. described two conscious, unanaesthetised subjects whose entire voluntarily (skeletal) musculature was paralysed with curare (except for one arm left intact so that the subject could make hand signals) and who were kept alive by mechanical ventilation. Turning off the ventilator did not induce any intense feeling of suffocation, nor distress, nor any urge to breathe. Fig. 1 shows that the two subjects remained unventilated for as long as the supervising anaesthetist permitted (about 4 minutes, when arterial PCO2 had reached ~72 mmHg) i.e.‘breath-hold’ duration was apparently prolonged indefinitely.
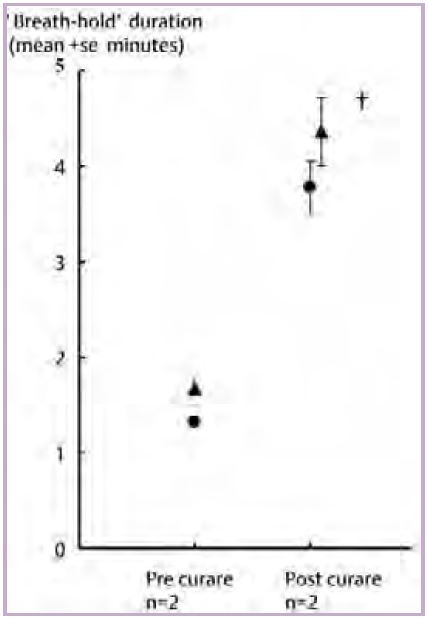
Is the mechanism explaining this astonishing observation simply curare preventing the diaphragm from contracting and hence its blood flow is never restricted sufficiently to activate diaphragm chemo-receptors? Unfortunately, the results of this intriguing and alarming experiment have not yet been confirmed. The one attempt to do so (Gandevia et al. 1993) was unsuccessful, but may be inconclusive since the three subjects apparently had had enough before their PCO2 levels had even risen above normal levels (43 mmHg).
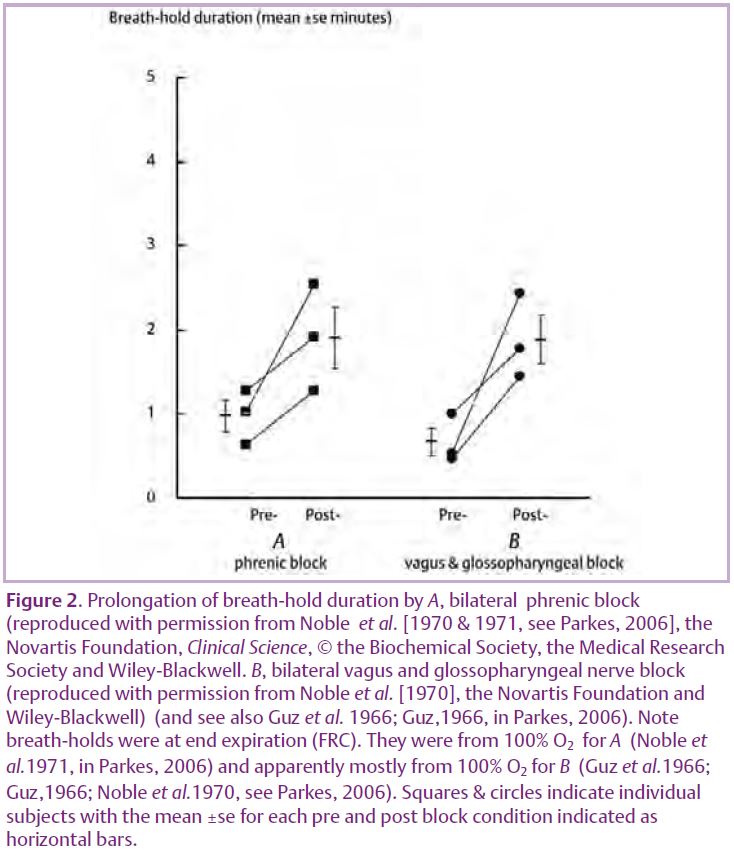
Most of Campbell’s results are, however, confirmed in a related experiment. Noble et al.(1970) locally anaesthetised the phrenic nerves bilaterally in three subjects. Such anaesthesia blocks the well known motor efferents to the diaphragm. It also blocks the rarely considered but extensive sensory afferents in the phrenic nerve that include those from diaphragm muscle chemoreceptors (Parkes, 2006). Blocking either would result in the loss of afferent activity from diaphragm chemoreceptors. Fig. 2A shows that Noble et al. (1970) confirmed a prolongation (doubling) of breath-hold duration.
Unfortunately these experiments, too, are now ethically unrepeatable. So it is unclear whether their failure to confirm Campbell’s indefinite ‘breath-hold’ duration is due to incomplete blockade of phrenic motor efferents and or sensory afferents, or that Campbell’s result is indeed unconfirmable.
The third less well known breath-hold prolonging manoeuvre is the demonstration (Fig. 2B) by Noble et al. (1970) (now also ethically unrepeatable) that bilateral local anaesthesia of the vagus and glossopharyngeal nerves prolongs (almost trebles) breath-hold duration. The precise afferents involved are unclear, but must involve blockade of non-pulmonary afferents (because pulmonary denervation itself has no effect on breath-hold duration (Flume et al. 1996; Harty et al. 1996, in Parkes, 2006) and blockade of non-carotid and non-aortic chemoreceptor afferents (because carotid denervation itself has no effect on breath-hold duration under similar hyperoxic conditions (Gross et al.1976, in Parkes, 2006) and aortic chemoreceptors have no effect on breathing in humans).
The breath-hold prolonging effects of diaphragm paralysis, phrenic and vagus with glossopharyngeal nerve blockade could be combined in the radical suggestion (Parkes, 2006) that diaphragm afferents might travel partly with or within the vagus nerves. This idea is not without precedent, since the vagus and phrenic nerves are sometimes in close proximity and the phrenic nerve in dogs does contains abdominal and thoracic afferents from the heart and vena cava (Kostreva & Pontus, 1993a; Kostreva & Pontus, 1993b).
Clearly the hypothesis that diaphragm chemoreceptors play a role on the breakpoint of breath-holding is still largely speculative. During breath-holding in man we still know almost nothing about diaphragm activity, nor its perfusion nor its chemoreceptor activity. Perhaps the latest developments in imaging will rekindle some interest in studying the mechanism explaining the breakpoint of breath-holding in man?
References
Gandevia SC, Killian KJ, McKenzie DK, Crawford M, Allen GM, Gorman RB & Hales JP (1993). Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol 470, 85-107.
Kostreva DR & Pontus SP (1993a). Hepatic vein, hepatic parenchymal, and inferior vena caval mechanoreceptors with phrenic afferents. Am J Physiol 265, G15-G20.
Kostreva DR & Pontus SP (1993b). Pericardial mechanoreceptors with phrenic afferents. Am J Physiol 264, H1836-H1846.
Noble MIM, Eisele JH, Trenchard D & Guz A (1970). Effect of selective peripheral nerve blocks on respiratory sensations. In Breathing: Hering-Breuer Centenary Symposium, ed. Porter R, pp 233-247. J & A Churchill (Longmans Group), London.
Parkes MJ (2006). Breath-holding and its breakpoint. Exp Physiol 91, 1-15.
