
Physiology News Magazine
Spontaneous transient currents, long slow oscillations… or just wobbles?
Len Best offers a cautionary tale of the elusive nature of an electrophysiological phenomenon
Spontaneous transient currents, long slow oscillations… or just wobbles?
Len Best offers a cautionary tale of the elusive nature of an electrophysiological phenomenon
Len Best
Department of Medicine, University of Manchester, UK
https://doi.org/10.36866/pn.60.30

As a non-physiologist by training (I must admit to being a reformed biochemist) I came to electrophysiology late. But once I’d tried it, I was hooked. One of the great attractions to me of electrophysiology was that you got your results (or lack of) during the course of your experiments. You could actually sit and watch your cells doing their thing – as if still inside the body – in real time. By contrast, biochemistry (or at least the sort of biochemistry I had been doing) tended to yield results up to a week after completion of the experiment (if at all), by which time I had usually realised that I had done the wrong experiment anyway, or lost interest for some other reason. But electro-physiology was different. It was … exciting.
So approaching my, er, ‘middle years’, I decided to arrange a sabbatical in the lab of an esteemed pancreatic islet cell electrophysiologist – name omitted to spare him embarrassment – in order to learn enough about patch-clamping to enable me to re-badge myself and make a start in this general direction. I learned how to fabricate and fill a patch pipette, what sort of ingredients the pipette and bath solution might typically contain and how to make those all-important seals. At this point, I thought I had it cracked. This was in spite of the fact that my mentor had pointed out on numerous occasions that the main difficulty in patchclamping was interpreting one’s experimental data. After all, wasn’t this true of just about everything?
In hindsight, I should have taken more heed of this sound advice. For example, it might have been advisable to begin working on a channel/current that was already well-characterised. The islet β-cell KATP channel was an obvious candidate. Adding to the already considerable wealth of information on this channel might have been the prudent option, with the added attraction of being able to talk to other electrophysiologists without them giving me funny looks and – who knows – maybe an improved chance of funding. But did I really want to be just another individual working on KATP channels? No, I would Do Something Different.
I had (and, to be honest, still have) a mild obsession with anion channels. I won’t bore you with the background to this obsession now – maybe another time. The received wisdom among the islet cell fraternity at the time (early 90s) was that, in the pancreatic islet βcell, chloride was at equilibrium. So even if anion channels were present in the β-cell, activating them would have virtually no effect on membrane potential or electrical activity. In any case, the received wisdom also stated that β-cells didn’t have anion channels. A likely explanation for this latter notion, it now appears, is that nobody had actually bothered to look for them. So the quest for islet cell anion channels began.
But how to start? The most obvious approach at the time seemed to be to block all the other channels I could think of and see what was left. A series of bizarre pipette solutions were made up, containing no sodium, potassium or calcium. The first series of experiments would be conventional whole-cell recordings – a kind of ‘catch-all’ for any remaining currents. From virtually the first cell on the first day, the results were startling. Or at least, I thought so. A pattern of long, slow, transient oscillating currents of variable amplitude and duration was apparent (see Fig. 1). This was it! I had discovered a ‘hidden current’ and perhaps, in doing so, solved the riddle of β-cell electrophysiology! Surely, these current oscillations must play some role in determining the oscillating pattern of β-cell electrical activity? I was therefore initially puzzled that touting my prized newly-obtained recordings around the lab failed to produce the anticipated excitement among my colleagues. Their reactions ranged from scepticism to utter bewilderment. The kindest remarks were along the lines of ‘Where’s zero current?’, ‘What’s the reversal potential?’ and ‘Have you got EGTA in your pipette solution?’ My initial heady enthusiasm rapidly subsided and I began to realise that these remarks were, in fact, rather helpful. Indeed, calcium-activated spontaneous transient inward (STICs) and outward (STOCs) currents were already well-documented, notably by William Large and colleagues.
But it turned out (again, I’ll spare you the details) that my currents were probably neither STICs nor STOCs. For one thing, it seemed almost impossible to block them. It was also difficult to shift their reversal potential in any meaningful or predictable manner. It was suggested to me that they might represent some kind of ‘seal phenomenon’. But this did not appear to explain the fact that pulling off an excised (outside-out) patch resulted in a corresponding reduction in current amplitude (see Fig. 1).
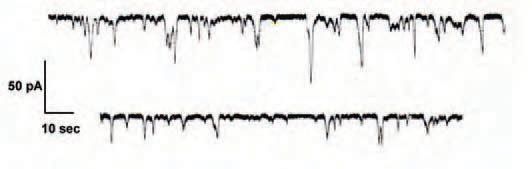
At this point, the current Editor of Physiology News, in a typically helpful and constructive moment, suggested the term ‘wobbles’ to describe my prized phenomenon. Undeterred, I presented my findings to the Physiological Society. To my great relief, among the sea of blank faces in the audience, one or two kind souls offered token questions. ‘Had I tried lanthanum?’ or ‘Had I noted any timedependent rundown?’ Still undeterred, I attempted to obtain funding to study this clearly important new conductance. To my complete astonishment, my application was declined. And then declined again. I was convinced I must really have struck on something of Titanic importance, and I resolved to pursue the matter To The Bitter End.
But the bitter end came sooner than I had anticipated, and in a rather different manner. My ‘wobbles’ disappeared literally overnight. I tried everything. Smaller electrodes, bigger electrodes, different shaped electrodes, adding ATP, adding lanthanum, heating the lab up, playing Led Zeppelin1 at top volume while attempting seal formation – but nothing.
Then, in an idle moment, I suddenly realised that the disappearance of the spontaneous currents had coincided with a seemingly minor change in the composition of our cell culture medium. Specifically, we were now using a new batch of fetal calf serum, having recently run out of our longterm favourite Canadian vintage.
So it would simply be a matter of obtaining more of the Canadian elixir, or at least finding a viable alternative. Well, no. In fact, neither option yielded any success whatsoever. The Canadian vintage was long sold-out, and no other fetal calf serum did the job. Wobbles weren’t just inexplicable – they were ephemeral too. And now they were lost in perpetuity. (Actually three bottles left in the freezer by a long-departed previous lab neighbour).
Well, not quite. To rub salt into the wound, my aforementioned sabbatical mentor later revealed to me during a bar session at an islet cell workshop that his lab too had observed ‘The Wobble Phenomenon’. ‘And’, he intimated ‘it was in one of the very best islet cell preparations we have ever made’.
A large drink (or several) seemed to offer the only possible consolation.
Len Best
Department of Medicine, University of Manchester, UK
P.S. It now turns out that chloride is not at equilibrium in the β-cell. And these cells express at least two types of (real!) chloride channel. But that’s another story. And I still have an unfinished manuscript on the
‘Wobbles’ in my bottom drawer …
