
Physiology News Magazine
Statins and implications for fetal brain sparing during hypoxia
Our time in the womb is not straightforward. As a growing fetus, our dividing cells require appropriate amounts of nutrients and oxygen. Limitations in this supply may lead to rapid deterioration in fetal wellbeing, which can be fatal or trigger serious, long-lasting consequences. Do statins help or harm the hypoxic fetus?
Features
Statins and implications for fetal brain sparing during hypoxia
Our time in the womb is not straightforward. As a growing fetus, our dividing cells require appropriate amounts of nutrients and oxygen. Limitations in this supply may lead to rapid deterioration in fetal wellbeing, which can be fatal or trigger serious, long-lasting consequences. Do statins help or harm the hypoxic fetus?
Features
Andrew Kane, Emilio Herrera, Avnesh Thakor & Dino Giussani
University of Cambridge, UK
https://doi.org/10.36866/pn.92.42
Sadly, despite advances in clinical obstetric practice, the occurrence of reductions in fetal oxygenation or hypoxia represents a common, serious challenge with significant chances of long term morbidity (e.g. hypoxic–ischaemic encephalopathy and cerebral palsy) or mortality for the neonate affected (Low, 2004). Fetal hypoxia in adverse pregnancy may arise during pre-eclampsia, placental insufficiency, placental abruption or umbilical cord occlusion. Fetal hypoxia may also occur, secondary to the composition of the maternal environment (e.g. high altitude, polluted air, smoke and carbon monoxide), maternal cardio-respiratory disease or as a result of maternal anaemia.
The strategy of an individual to withstand episodes of hypoxia differs in fetal and postnatal life. In the simplest terms, in the postnatal period, our physiological response to acute hypoxia is to increase our alveolar ventilation rate and cardiac output and decrease our peripheral vascular resistance, in an attempt to maintain blood oxygen delivery to our respiring tissues. However, the fetus has no such ability to increase pulmonary oxygenation and has to survive with any reduction in oxygen delivery imposed by the placenta or the maternal environment. Using the late gestation sheep fetus as the animal model of choice, it has been shown that the fetal strategy is to make best use of the available oxygen supply, redistributing cardiac output away from the peripheral organs, such as the gut and limbs, and towards more essential circulations, such as those perfusing the fetal brain (Cohn et al. 1974). This ‘brain sparing’ defence to acute hypoxia during fetal life is achieved through coordinated neural, endocrine and metabolic mechanisms. We know that the fetus can sense hypoxia via the carotid body chemoreceptors, and that this information is relayed to the fetal brain via the glossopharyngeal nerves (Giussani et al. 1993). In turn, there is activation of both the sympathetic and parasympathetic arms of the autonomic nervous system. The neural component of the sympathetic nervous system drives vasoconstriction of the peripheral circulation, hence increasing peripheral vascular resistance and reducing peripheral blood flow. In contrast, cerebral vascular resistance is decreased, directing a greater proportion of blood flow to the fetal brain (Rudolph, 1984; Giussani et al. 1993). If the period of hypoxia is prolonged and/or severe, the fetus will release a vast array of agents into the fetal circulation, including catecholamines, cortisol, angiotensin II, vasopressin and neuropeptide Y, which maintain peripheral vasoconstriction and, thereby, the redistribution of blood flow (Giussani et al. 1994). In addition, the fetus mounts a metabolic response. Hypoxia results in an increase in anaerobic respiration with less ATP generated per unit glucose. Therefore, elevations in fetal plasma catecholamine levels drive a hyperglycaemic response resulting from a decrease in glucose uptake and utilisation by peripheral tissues and an increase in hepatic glucose production by promoting glycogenolysis and gluconeogenesis (Jones, 1977; Jones et al. 1983). The fetal lactic acidaemia arises from anaerobic metabolism of glucose in hypoxic fetal tissues, particularly in the hind limbs where blood flow and oxygen delivery markedly decline (Boyle et al. 1990). Interestingly, many aspects of this fetal defence to hypoxia are well conserved across species, from reptiles to birds and mammals, including non-human primates and the human fetus (Giussani, 2006).

Recently, work in our laboratory has focused on the contribution of the fetal vasculature itself to the fetal redistribution of blood flow during acute hypoxia. In addition to neuroendocrine control, it is now recognised that the cellular oxidant milieu is an important modulator of vascular resistance (Chen & Keaney, 2004; Valko et al. 2007). In the adult vasculature it is established that there are increases in the production of the superoxide anion (·O2–), which will react with nitric oxide (NO), reducing its bioavailability. An increase in the vascular ratio of ·O2–:NO will thus promote vasoconstriction, and the reverse will favour vasodilatation. Reactive oxygen species (ROS) are generated through pro-oxidant systems including the mitochondrial electron transport chain, uncoupled eNOS, xanthine oxidase, NADPH oxidase and cytochrome P450. Under normal physiological conditions, ROS are continuously degraded by antioxidant defences including enzymatic disposal by superoxide dismutase, catalase and glutathione peroxidase, and/or by free-radical scavenging molecules such as vitamins C and E, melatonin and the carotenes (Valko et al. 2007). However, at higher concentrations, ·O2– may react with NO instead of being degraded or binding to an antioxidant molecule, thereby having implications for cardiovascular regulation. In the fetal circulation, it has been appreciated for some time that NO contributes to the maintenance of blood flow in many vascular beds, including the umbilical, cerebral, myocardial, femoral and carotid circulations, as inhibition of NO synthesis leads to pronounced increases in vascular resistance. It is also known that during acute hypoxia, enhanced NO opposes chemoreflex and endocrine vasoconstrictor influences in the femoral vascular bed, thereby fine-tuning the fetal peripheral vasoconstrictor response to hypoxia (Morrison et al. 2003). However, the role of free radicals and their interaction with NO in the control of the fetal circulation in health or disease had not been established until very recently.
Work in our laboratory has now shown that treatment of fetal sheep with the antioxidants vitamin C or melatonin, which are able to quench O2– in the circulation, promotes significant vasodilatation in the umbilical vascular bed, leading to significant increases in umbilical blood flow (Thakor et al. 2010a). In another study published in The Journal of Physiology, fetal treatment with vitamin C led to dilatation of the fetal femoral vasculature during basal conditions and impaired the fetal femoral constrictor response to acute hypoxia (Fig. 1; Thakor et al. 2010b). The data suggest that antioxidant sequestration of O2– within the fetal vasculature, and prevention of the reaction with NO, increase the bioavailability of NO, promoting vasodilatation and, thereby, increasing flow under basal conditions and opposing peripheral vasoconstrictor influences during stimulated conditions, such as during fetal hypoxia. This was later confirmed as fetal treatment with antioxidants in the presence of the NO clamp, an in vivo technique that blocks NO synthesis without affecting basal cardiovascular function (Gardner & Giussani, 2003), restored the magnitude of fetal peripheral vasoconstriction (Fig. 1).
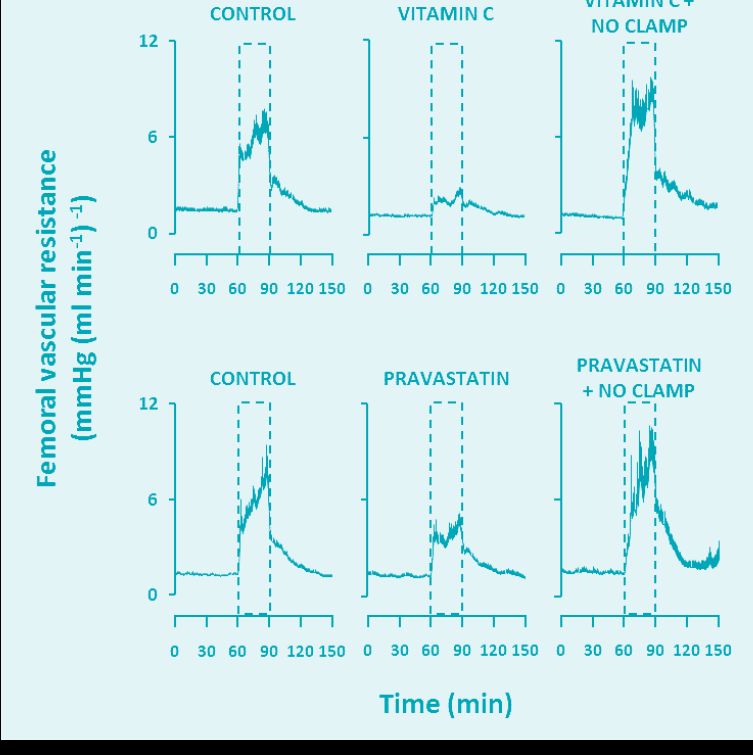
The discoveries of the operation of an oxidant tone in the fetal vasculature and its manipulation with antioxidants driving changes in blood flow have important implications for the use of drugs in pregnancy that increase NO bioavailability. One such example is HMG-CoA reductase inhibitors. Statins inhibit the rate-limiting step in cholesterol synthesis and have therefore become some of the most effective and widely prescribed drugs for the primary and secondary prevention of coronary heart disease (Steinberg, 2008). In addition to their lipid lowering action, additional beneficial effects on the circulation have been noted, including decreases in arterial stiffness, reductions in platelet aggregation and improvements in vascular endothelial function. These benefits have been credited to statin-induced increases in NO bioavailability and increased NO function through a variety of mechanisms (Adam & Laufs, 2008). Considering the rising levels of obesity and associated lipid disorders in younger populations (National Center for Disease Statistics, 2011) and that women are delaying childbirth until the fourth or fifth decades of life (Heffner, 2004), there is growing clinical interest in being able to treat pregnant women with statins, if required. Indeed, one large randomised multi-centre clinical trial has begun recruiting patients in the United Kingdom to investigate if pravastatin could reduce circulating anti-angiogenic factors associated with pre-eclampsia (the ‘StAmP’ trial; Ahmed, 2011). In another recent study published in The Journal of Physiology, the fetal femoral vasoconstrictor response to acute hypoxia was assessed under control conditions, and following treatment with a clinically relevant dose of pravastatin (Kane et al. 2012). The experiments demonstrated that fetal exposure to pravastatin depressed the fetal peripheral vasoconstrictor responses to acute hypoxia (see Fig. 1). Further, these effects could be prevented in fetal sheep treated with pravastatin under NO clamp conditions, demonstrating that increases in NO levels under pravastatin treatment contributed to the suppression in the femoral vasoconstriction to hypoxia. The data support the hypothesis that statins increase NO bioavailability and oppose neuro-endocrine influences that mediate the peripheral vasoconstriction and metabolic responses to hypoxic stress in the fetus.
At first sight, the results appear concerning given the clinical interest in using statins in complicated pregnancy. Statins may impair the fetal brain sparing response to birth hypoxia. However, the maintenance or increase in cerebral blood flow and, thereby, cerebral oxygen and nutrient delivery, which spares the fetal brain during episodes of hypoxia or asphyxia, is not only dependent on vasoconstriction in the peripheral vascular beds, but also on active vasodilatation in the cerebral circulation. Indeed, this is mediated by mechanisms involving increased NO (Green et al. 1996), and several studies have reported a maintained increase in cerebrovascular perfusion during acute hypoxia even in the complete absence of peripheral vasoconstriction, for instance with carotid sinus nerve denervation or α1 adrenergic blockade (Giussani et al. 1993). Therefore, in circulations which constrict, such as the femoral vascular beds, enhanced NO bioavailability may diminish peripheral vasoconstriction. However, in circulations which dilate particularly via NO-dependent mechanisms during acute hypoxia, such as the cerebral vascular bed, enhanced NO bioavailability may actually increase cerebral blood flow. Therefore under conditions of fetal exposure to statins or antioxidants, the fetal cardiovascular strategy to defend against hypoxia may change to increase cardiac output and maintain perfusion to most circulations. Clearly, there is an urgent need to assess the impact of antioxidant or statin exposure on changes in fetal cerebral blood flow and oxygen delivery as well as in the fetal peripheral circulations during acute fetal hypoxia. For now, we propose that the use of statins or antioxidants in pregnancy should be considered with extreme caution.
References
Adam O & Laufs U (2008). Antioxidant effects of statins. Arch Toxicol 82, 885–892.
Ahmed A (2011). New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thrombosis Research: Papers and Abstracts of The 4th International Symposium on Women’s Health Issues in Thrombosis and Haemostasis 127, S72–S75.
Boyle DW, Hirst K, Zerbe GO, Meschia G & Wilkening RB (1990). Fetal hind limb oxygen consumption and blood flow during acute graded hypoxia. Pediatr Res 28, 94–100.
Chen K & Keaney JF (2004). Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium 11, 109–121.
Cohn HE, Sacks EJ, Heymann MA & Rudolph AM(1974). Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120, 817–824.
Gardner DS & Giussani DA (2003). Enhanced umbilical blood flow during acute hypoxemia after chronic umbilical cord compression: a role for nitric oxide. Circulation 108, 331–335.
Giussani DA (2006). Prenatal hypoxia: Relevance to developmental origins of health and disease. In Developmental Origins of Health and Disease, ed. Gluckman PD & Hanson MA, pp. 178–190. Cambridge University Press, Cambridge.
Giussani DA, Spencer JA & Hanson MA (1994). Fetal cardiovascular reflex responses to acute hypoxaemia. Fetal Matern Med Rev 6 17–37.
Giussani DA, Spencer JA, Moore PJ, Bennet L & Hanson MA (1993). Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461, 431–449.
Green LR, Bennet L & Hanson MA (1996). The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol 497, 271–277.
Heffner LJ (2004). Advanced maternal age – how old is too old? N Engl J Med 351, 1927–1929.
Jones CT (1977). The development of some metabolic responses to hypoxia in the foetal sheep. J Physiol 265, 743–762.
Jones CT, Ritchie JW & Walker D (1983). The effects of hypoxia on glucose turnover in the fetal sheep. J Dev Physiol 5, 223–235.
Kane AD, Herrera EA, Hansell JA & Giussani DA (2012). Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol 590, 323–334.
Low JA (2004). Reflections on the occurrence and significance of antepartum fetal asphyxia. Best Pract Res Clin Obstet Gynaecol 18, 375–382.
Morrison S, Gardner DS, Fletcher AJ, Bloomfield MR & Giussani DA (2003). Enhanced nitric oxide activity offsets peripheral vasoconstriction during acute hypoxaemia via chemoreflex and adrenomedullary actions in the sheep fetus. J Physiol 547, 283–291.
National Center for Health Statistics (2011). Health, United States, 2010: With Special Feature on Death and Dying. National Center for Health Statistics , Hyattsville, MD, USA.
Rudolph AM (1984). The fetal circulation and its response to stress. J Dev Physiol 6, 11–19.
Steinberg D (2008). The statins in preventive cardiology. N Engl J Med 359, 1426–1427.
Thakor AS, Herrera EA, Serón-Ferré M & Giussani DA (2010a). Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res 49, 399–406.
Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L & Giussani DA (2010b). Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol 588, 4235–4247.
Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M & Telser J (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39, 44–84.
