
Physiology News Magazine
Stem cells for the treatment of Parkinson’s disease
The potential of stem cells in regenerative medicine is huge. The challenge is to understand how they can be directed and controlled.
Features
Stem cells for the treatment of Parkinson’s disease
The potential of stem cells in regenerative medicine is huge. The challenge is to understand how they can be directed and controlled.
Features
Rupert CM Wright, Paul Roach & Rosemary A Fricker
Keele University
https://doi.org/10.36866/pn.89.30
Many tissues in the human body such as nerve cells and cardiac muscle do not have a strong ability to regenerate when damaged. With ageing populations where degenerative disease is common, healthcare systems are under pressure, so regenerative medicine is being developed using cells to restore diseased or damaged tissue.
To date, cell therapies for neurodegenerative disorders such as Parkinson’s have been attempted in small clinical trials, using grafts of dopamine-producing neurons directly dissected from fetal central nervous system (CNS) tissue (Lindvall & Björklund, 2004). These trials have shown the potential of cell replacement therapies; however, the cell source is scarce and is insufficient to cover the demands of the patient population. To circumvent the need for large numbers of cells, stem cells are proposed as a more suitable source for neural transplants. Here we outline the key innovations and challenges surrounding stem cell therapies for neurodegenerative disease, focusing specifically on advances in Parkinson’s.
Which stem cells to use in cell therapies?
Stem cells are found throughout the body from development through to adulthood. They are defined by two main properties: self-renewal and the ability to become (differentiate into) mature cell types.
Stem cells range from being:
• Pluripotent – can differentiate into any cell type in the body.
• Multipotent – have a more restricted lineage, e.g. neural stem cells form neurons and glia of the (CNS).
• Unipotent/bipotent – make one or two cell types, respectively.
Stem cell therapies branch in two main forms:
Autologous: Same donor and recipient. This is a personalized medicine that avoids immune rejection. Stem cells can either be re-programmed in situ or removed from a patient, manipulated and returned in a transplant.
Allogeneic: Different donor and recipient. The advantage here is that these could be derived from more diverse sources, e.g. embryonic stem cells. Matching will be required for immune compatibility: it is estimated that a cell bank of 150 embryonic stem cell lines would be required to cover 85% of the British population (Taylor et al. 2010).
Identification is only the first step to achieving high yields of specific cell types from stem cells. A major challenge with developing optimal cells for therapies is recreating the complex spatial and temporal signalling processes that are required to create specific mature cells (e.g. neurons) within organised three-dimensional tissues and structures.
Embryonic stem cells
Embryonic stem cells (ESCs) are pluripotent, forming any cell type in the body, and show extensive self-renewal meaning large clonal populations can be produced. ESCs were first separated from mice (Evans & Kaufman, 1981), and more recently from human embryos (Thomson et al. 1998), derived from the inner cell mass of blastocysts provided by residual IVF tissue. This makes their use contentious for some, whilst others argue that the potential merits (generating a limitless number of cells from a small starting source) outweigh the ethical concerns of blastocyst destruction. ESCs can be steered into many mature cell lineages with appropriate cues.
Dopaminergic neurons have been derived from embryonic stem cells using a variety of culture methods. Effective methods include transfection with genes known to enhance neuronal differentiation (intrinsic signals) and the use of co-cultures or the addition of signalling molecules (cytokines, mitogens, trophic factors or morphogens) in a complex culture medium, to mimic the cells’ natural environment (extrinsic signals).
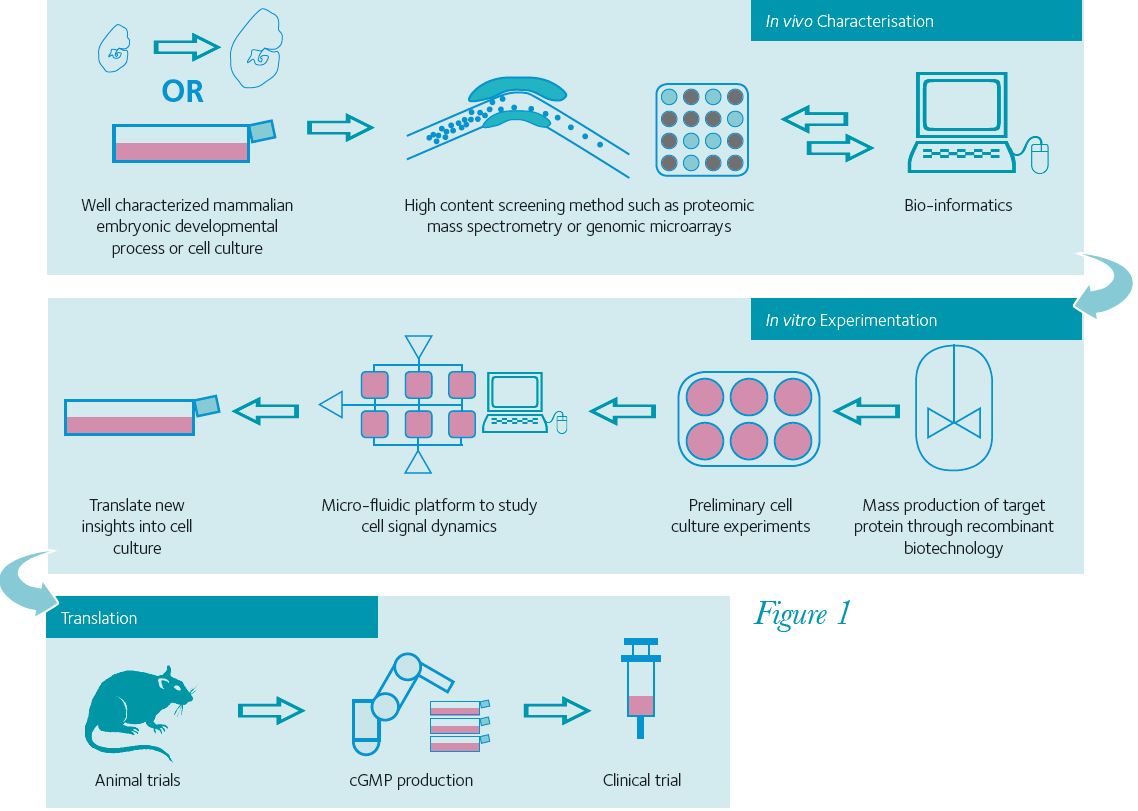
The task of finding relevant signal molecules has become more efficient as a result of high-throughput gene and protein screening methods such as genetic micro-array technology and proteomic mass spectrometry (Fig. 1). Our research group has utilised a mass spectrometry method to find new molecules to detect extrinsic signals (Orme et al. 2010). Proteins were harvested from developing midbrain tissue, targeting our search for specific protein signalling molecules by comparing their temporal and regional expression. Thus, we have identified novel and/or previously unidentified proteins that play key roles in the differentiation of dopamine neurons. Using genomic micro-arrays it is possible to look for gene products coding for intracellular signalling molecules to identify key transcription factors for defining neural lineages (Panman et al. 2011).
Some of the challenges of working with ESCs have already been overcome. Early problems such as karyotype abnormalities (incorrect numbers of chromosomes) have been avoided through stable culture protocols. Also, defined environments have been developed for human ESC culture, to remove the risk of animal/human pathogen transmission from media components or substrates.
Adult stem cells
Adult stem cells have fewer ethical hurdles because no embryos are destroyed during their production. Adult stem cells have been used extensively by clinical professionals, e.g. stromal bone marrow and adipose tissue to provide mesenchymal stem cells (MSCs) that are multipotent. When a patient is given a bone marrow transplant for leukaemia, the bone marrow stem cells replenish the recipient’s supply of haematopoietic stem cells.
The adult CNS contains two distinct but small pools of neural stem cells, with the potential to treat Parkinson’s. However, in vitro it is difficult to derive dopaminergic neurons from adult neural stem cells and the cells are difficult to harvest. Through a process called transdifferentiation where a cell’s lineage changes, dopaminergic neurons have been derived from bone marrow stem cells. The problem is that the cells express a lot of relevant markers but do not function as mature neurons, rendering these cells unsuitable for therapies.
Adult stems cells are safest, but currently have a limited potential. One problem is generation of large numbers of relevant cell types as the fates of the adult stem cells are often restricted; another problem is that it is difficult to expand the cells into large populations for clinical use.
Induced pluripotent stem cells
In 2006, Nobel prize winning research led to a new breakthrough – generation of induced pluripotent stem cells (iPSCs), derived from mature tissues (mouse fibroblast cells) and reprogrammed to a stem cell state using key pluripotency genes (Takahashi & Yamanaka, 2006). iPSCs have similar properties to ESCs but not the ethical problems associated with destruction of embryos.
The promise of iPSCS for the treatment of neurodegenerative disease has been demonstrated by their conversion to neurons, shown to improve movement problems following transplantation to models of Parkinson’s (Soldner et al. 2009).
A number of research groups have now derived iPSCs successfully from Parkinson’s patients, thus generating autologous cell types for transplantation or for disease modelling (Soldner et al. 2009). In further technological developments, there is some evidence to suggest that it is possible to derive neurons directly from mature tissues, omitting the naïve stem cell stages (Caiazzo et al. 2011). Stem cell-derived disease models would be a fantastic tool for the pharmaceutical industry and biotech companies, to test promising molecular/gene therapies.
Safety concerns have been raised over the reprogramming factors used to generate iPSCs and their delivery using viruses. However, more recent work suggests that iPSCs can be induced without using genes associated with tumour formation, and that reprogramming can be accomplished more safely, using excisable viruses, or avoiding DNA or viruses altogether by using chemically modified proteins.
However, iPSCs have been found to be less efficient than ESCs in differentiating into neurons (Chin et al. 2009), probably due to genetic and epigenetic differences. Another concern is that iPSCs are not truly naïve but possess latent ‘memories’ of their original lineage e.g. an iPSC derived from blood cells is more likely to turn into a haematopoietic cell than a neuron.
The complexity of stem cell differentiation
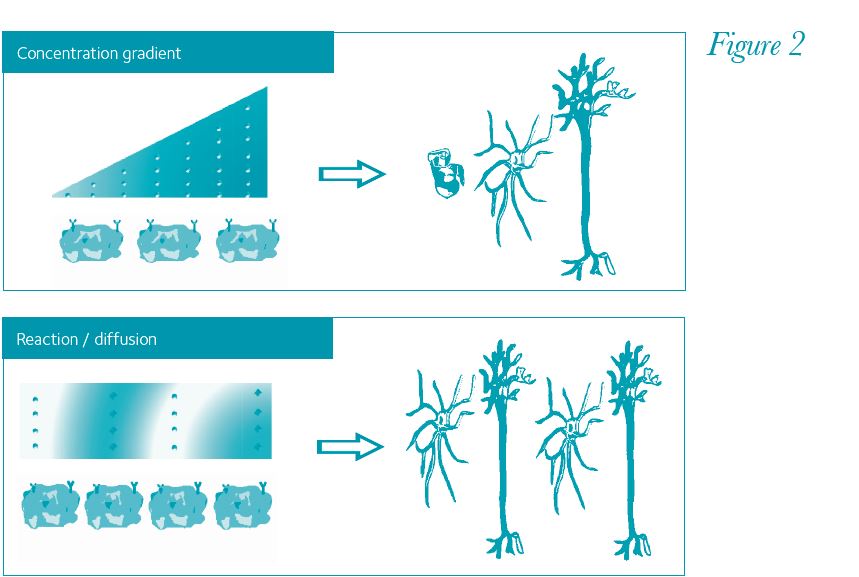
Currently one of the biggest challenges with stem cells is to differentiate them with high efficiency and create complex structures (Fig. 1). Some recent achievements might indicate that technology is improving: highly functional dopamine neurons have recently been derived from ESCs in vitro, following developmental principles (Kriks et al. 2011; Jaeger et al. 2011). The key to success seems to be providing the cells with sequential and specific signal molecules that are up-regulated at different times in the development of dopamine neurons. With so many signal molecules, the challenge is to figure out the dynamics of how stem cells experience signal molecules during their differentiation. The simplest dynamic is that different concentrations of signal molecules will push naïve stem cells down different lineages. Good examples are sonic hedgehog and Wnt proteins that work in a concentration gradient during neural tube development. More complex spatial patterns can be described with Alan Turing’s reaction/diffusion model (Turing, 1952; Fig. 2), where activator and inhibitor signals spontaneously organize into binary patterns. At the single cell level, some signal molecules can elicit greater responses when delivered as a pulse rather than a steady dose (Ashall et al. 2009). Also, heterogeneous cell responses can occur to the same stimuli through internal feedback loops within individual cells and interplay between neighbouring cells. These complex dynamics could be responsible for the lack of effectiveness in current differentiation protocols. There is a non-linearity to developmental systems and its modelling is likely to be important for future stem cell research. There are multiple opportunities to integrate computational methods into stem cell science, for instance incorporating microfluidic designs to differentiation protocols (Fig. 1, middle panel). These advances will provide better understanding of cellular systems and improve the efficiency of stem cell differentiation, a crucial step for their translation towards clinical therapies.
References
Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K et al. (2009). Pulsatile stimulation determines timing and specificity of NF-β-dependent transcription. Science 324, 242–246.
Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D et al. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227.
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C et al. (2009). Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5, 111–123.
Evans MJ & Kaufman MH (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156.
Jaeger I, Arber C, Risner-Janiczek JR, Kuechler J, Pritzsche D, Chen I-C et al. (2011). Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development 138, 4363–4374.
Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551.
Lindvall O & Björklund A (2004). Cell therapy in Parkinson’s disease. NeuroRx 1, 382–393.
Orme RP, Gates MA & Fricker-Gates RA (2010). A multiplexed quantitative proteomics approach for investigating protein expression in the developing central nervous system. J Neurosci Methods 191, 75–82.
Panman L, Andersson E, Alekseenko Z, Hedlund E, Kee N, Mong J et al. (2011). Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell 8, 663–675.
Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG et al. (2009). Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977.
Takahashi K & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676.
Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA & Bradley JA (2010). Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet 366, 2019–2025.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS & Jones JM (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147.
Turing AM (1952). The chemical basis of morphogenesis. Phil Trans R Soc Lond B 237, 37–72.
