Physiology News Magazine
Stress and the gut – it’s not all in your mind
How interactions between the brain, the gastrointestinal system, and the microbial residents of the gut influence both gastrointestinal and cognitive function
Features
Stress and the gut – it’s not all in your mind
How interactions between the brain, the gastrointestinal system, and the microbial residents of the gut influence both gastrointestinal and cognitive function
Features
Kim E. Barrett
Division of Gastroenterology, Department of Medicine, University of California, USA
https://doi.org/10.36866/pn.108.28
We are all intuitively aware that stress has an impact on the function of our digestive system. Whether it is the transient butterflies that accompany an acute stressor such as an exam or an interview, or the more toxic consequences of chronic stress for gut function, our cognitive experience of our world and its challenges can profoundly alter our physiological functions of digestion, absorption, and excretion. What has been less fully appreciated until recently, however, is that communication between the brain and gut is bi-directional, and gastrointestinal illnesses may be accompanied by neuropsychiatric disorders, such anxiety, depression, and memory deficits. The central nervous system and the gastrointestinal system are in constant communication, in part via the enteric nervous system or ‘little brain’ of the gut. We are also rapidly learning of the ways in which the microbes that reside in our gut may be important mediators of the cross-talk between gut and brain. They are, in turn, influenced by environmental conditions, such as stress and diet, in ways that modulate their impact on both digestive and cognitive function.

As humans, we like to think that we are masters of our own universe. But in fact, it has now become abundantly apparent that we, along with all other beings, are actually superorganisms. Thus, we consist not only of our own cells and genome, but also of distinctive populations of symbiotic microbes, along with their associated genetic material and repertoire of metabolic capabilities. This so-called ‘microbiota’ is comprised prominently of bacteria, which have been the best-studied populations, but also of fungi, bacteriophages and other viruses, and archaea, which are only now beginning to be examined. Specialised microbiota inhabit a variety of body niches, such as the intestines, oral cavity, skin, and respiratory and genital tracts, although the most extensively characterised of these is the gut-associated bacterial microbiota, consisting of thousands of unique species in a typical healthy human adult. The gut microbiota resides throughout the length of the intestine, but is most heavily concentrated in the colon. Estimates of the number of gut bacteria suggest that there are as many as 1014 throughout the gut, or 10 times the number of human cells in the entire body (Sekirov et al., 2010). Recently, the 10:1 ratio has been disputed, with a claim that the numbers of human cells and gut bacteria are of the same order of magnitude (Sender et al., 2016). But even if this is true, the microbes collectively encode significantly more genes than the cells of their host.
Our symbiotic microbes – what do they do for us?
As illustrated by the ability to raise experimental animals in a germ-free state, the microbiota, in the gut or elsewhere, is not essential for life. Nevertheless, the gut microbiota in particular offers several advantages to the host. It embodies a large capacity for metabolic conversion of ingested nutrients that cannot otherwise be assimilated by the host, such as dietary fibre, as well as the metabolism of xenobiotics and vitamin precursors. Similarly, the microbiota is said to ‘educate’ the mucosal immune system, either directly or by producing immunoregulatory metabolites, ensuring (at least in health) that the gut is largely tolerant of its resident commensal organisms as well as innocuous dietary proteins, while remaining on-guard to protect against pathogens. The gut microbiota also contributes to the homeostasis of the epithelial lining as well as its barrier function, and appears to support the development of the microvasculature, acquisition of appropriate motility responses, and maturation of the enteric nervous system (Obata & Pachnis, 2016). The organisms of the microbiota also defend against colonisation with pathogens, either by direct physical exclusion, by producing bacteriostatic or bacteriocidal products, or by stimulating the host to secrete antimicrobial peptides. Indeed, a course of broad-spectrum antibiotics given for an extraintestinal disease, such as a chest infection, can render patients susceptible to the consequences of intestinal infections and/or overgrowth of injurious bacteria, such as Clostridium difficile (C. diff).
It is commonly held that the gut microbiota begins to establish itself immediately after birth, notwithstanding some controversial data that suggest the presence of microbes in the fetus in utero. Indeed, the ability to derive germ-free animals via Caesarean section (C-section) implies that if microbial DNA is in fact present in the womb, it likely does not reflect the presence of viable organisms (Perez-Munoz et al., 2017).
What is well-established is that the initial gut microbiota shares many characteristics of the mother’s vaginal microbiota for babies delivered vaginally, and differs substantially for those delivered by C-section. An intriguing recent study partially restored a ‘normal’ microbiota in the gut, oral cavity, and skin by exposing babies delivered by C-section to the mother’s vaginal fluids, with the authors speculating that this might reverse the known association between C-section deliveries and an increased risk for immune and metabolic disorders (Dominguez-Bello et al., 2016). After birth, the baby’s microbiota is relatively simple and variable for the first year or two of life, but gradually takes on the characteristics of a mature, adult-like microbiota. In that this is also a critical period for maturation of the immune system, it is perhaps not surprising that disruptions in the normal process for parallel maturation of the microbiota are felt to predisposeto autoimmune and allergic diseases. For example, the increasing tendency to protect infants from microbial exposure may set them up for an increased later risk of asthma, metabolic disease, or obesity (the ‘hygiene hypothesis’), as does excessive/indiscriminate early-life use of antibiotics (Schulfer & Blaser, 2015).
The microbiota as a mediator of responses to stress
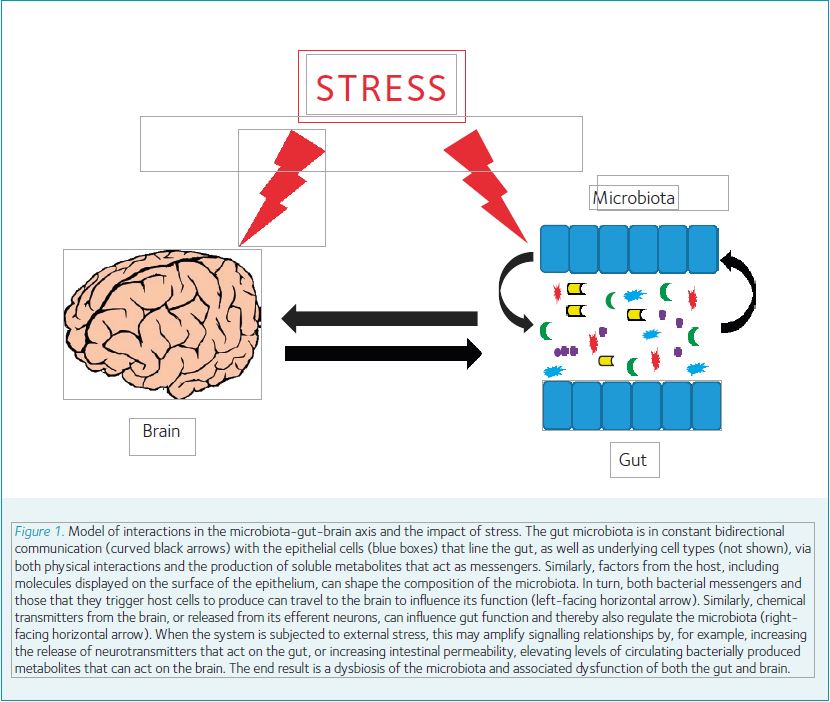
But how does all of this relate to stress,The Society’s theme for 2017 and the impetus for the invitation to write this article? The gut microbiota perhaps predictably shows alterations in the setting of a variety of intestinal disease states, such as inflammatory bowel diseases and irritable bowel syndrome, and indeed, some of the characteristics of these diseases can be transferred to naïve, previously germ-free animals with the microbiota from affected mice or humans. But it is also becoming increasingly clear that gut microbes and their products may have effects well beyond the confines of the intestine itself. Perhaps the most intriguing aspects of this area of research relate to observations that tie the composition of the gut microbiota to cognitive function and/or the cognitive response to stress. Further, the adverse effects of stressful stimuli on gut function depend on the presence of intestinal microbes. The experimental findings reported to date support the model of a bi-directional microbiota-gut-brain axis (Fig. 1) that influences the normal function of both bowel and brain alike, and may explain, for example, the comorbidity of specific digestive and psychiatric disorders (Gareau, 2016). And while the evidence in human patients is largely correlative at present, it is intriguing to observe that derangements in the microbiota have been associated with numerous neuropsychiatric conditions, including depression, autism, schizophrenia, and perhaps even Parkinson’s disease (Dinan & Cryan, 2017).
Therapeutic manipulation of the microbiota – from probiotics to transplants
So if both intestinal and neuropsychiatric conditions are potentially attributable to alterations in the gut microbiota (at least in part), and if such alterations also mediate the impact of stress on the relevant organ systems, can we mitigate these outcomes by targeting the microbiota? Several approaches have been posited to have either beneficial or deleterious effects on the make-up of the gut microbiota and its intestinal and extraintestinal influences. Perhaps the most obvious of these is the diet. While the gut microbiota was at one time felt to be relatively immutable in adulthood, improved analytic approaches indicate that its make-up in fact is profoundly influenced by the composition of the diet and even by the timing of meals. For example, Western diets, high in meat and fat, decrease the diversity of the microbiota (and may even promote the emergence of pathogenic properties in commensals) whereas diets rich in plant-based fibre increase it. Another approach to targeting the microbiota is the use of antibiotics, although for the reasons discussed above these are likely to be deleterious in the main, particularly early in life. The composition of the microbiota can also be altered directly by the administration of probiotics, which are commensal micro-organisms selected for their apparent health benefits that can be taken orally. Studies in animal models demonstrate that probiotics can improve both gut and cognitive function in animals exposed to a variety of stressors, or can negate the cognitive dysfunction accompanying intestinal inflammation or infection. However, not all probiotics are created equal, and much work remains to be done both to validate animal studies in human clinical trials and to define characteristics of probiotic strains that predict efficacy in a given clinical setting.
Perhaps the approach to targeting the microbiota that has attracted the most recent public attention is the practice known as faecal microbial transplant (FMT), where faecal material is transferred from a healthy donor (often a relative) to someone suffering from a specific intestinal or extraintestinal disease. Enthusiasm for FMT derived initially from its dramatic efficacy in some patients suffering from disabling and persistent diarrheal disease as well as other symptoms associated with treatment-resistant C. diff infections. More recently, there have been encouraging data suggesting that FMT may be effective in producing remission in inflammatory bowel disease, although the long-term consequences are unknown and larger, well-controlled studies are needed. Exploratory reports even suggest beneficial effects of FMT on gastrointestinal and behavioural symptoms of autism, or in obesity and metabolic syndrome, but much further work is needed to validate these preliminary data. Ideally, FMT procedures should be conducted under carefully–controlled and physician-supervised conditions to screen for the potential presence of pathogens or toxins. Nevertheless, despite the obvious ‘yuck’ factor, ‘do-it-yourself’ instructions can readily be found online (there are even Facebook groups), and some individuals are sufficiently distressed by their condition to give it a try.
Closing thoughts – mitigating negative effects of stress
In conclusion, therefore, it is clear that our response to stress, whether manifested in our thought patterns or in our gut, is dramatically shaped by the microbiota that resides in the intestines. Particularly in humans, studies conducted to date have largely been confined to cataloguing the key players in a given setting, but animal data are provocative, and functional studies in humans will doubtless follow. No matter what, in the coming years, the explosive growth of studies aiming to target the microbiota for health benefits should give us a much better understanding of which approach, if any, is likely to be most beneficial for a given condition and even a given individual, since host factors clearly can also impact our microbial composition. This work holds the promise of ameliorating negative effects of stress, and perhaps may offer new avenues for the therapy or even prevention of the myriad of stress-related disease states that are increasing in incidence in developed countries.
The choice of citations was intended chiefly to be illustrative of selected recent contributions to the field. The author apologises to the many colleagues whose seminal works could not specifically be cited in this informal article, due to space limitations.
References
Dinan TG & Cryan JF (2017). Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595, 489–503.
Dominguez-Bello MG et al. (2016). Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22, 250–253.
Gareau MG (2016). Cognitive function and the microbiome. Int Rev Neurobiol 131, 227–246.
Obata Y & Pachnis V (2016). The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151, 836–844.
Perez-Munoz ME et al. (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48.
Schulfer A & Blaser MJ (2015). Risks of antibiotic exposures early in life on the developing microbiome. PLoS Pathog 11, e1004903.
Sekirov I et al. (2010). Gut microbiota in health and disease. Physiol Rev 90, 859–904.
Sender R et al. (2016). Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14, e1002533.