Physiology News Magazine
Surviving life in the womb and the implications for vascular health in adulthood
The early environment of the fetus can shape its risk for cardiovascular disease in adulthood. Females, by and large, fare better than males following these early life insults. However, changes in the function of the uterine vessels may predispose to pregnancy complications and perpetuation of cardiovascular disease risk in the next generation.
Features
Surviving life in the womb and the implications for vascular health in adulthood
The early environment of the fetus can shape its risk for cardiovascular disease in adulthood. Females, by and large, fare better than males following these early life insults. However, changes in the function of the uterine vessels may predispose to pregnancy complications and perpetuation of cardiovascular disease risk in the next generation.
Features
Marianne Tare (1), Marc Q. Mazzuca (2), Helena C. Parkington (1), Nicoleta Dragomir (3,4) and Mary E. Wlodek (2)
1: Department of Physiology, Monash University, Victoria, Australia
2: Department of Physiology and 3: School of Physics, The University of Melbourne, Victoria, Australia
4: School of Biomedical and Health Sciences, Victoria University, Victoria, Australia
https://doi.org/10.36866/pn.83.29

The womb is not necessarily a cosy and nurturing environment, as one might expect. Although the fetus may be endowed with genes bestowing physiological fortitude and resilience, the environment in the womb can sculpt the physiological phenotype and ultimately influence the risk of disease. The early studies by McCance and Widdowson in the 1960s–1970s laid the scientifi c foundations that life in utero can infl uence, for better or for worse, the ability of the offspring to achieve its full genetic potential after birth (McCance & Widdowson, 1974; Bateson, 2001). In the 1980s the seminal work of Barker and colleagues linked slowed human fetal growth to an increased risk of cardiovascular and metabolic disease in adulthood (Barker, 1995), and this was the catalyst for an intensive research focus into what is now known as the ‘developmental origins of health and disease’ hypothesis.
Life in the womb can be a struggle, especially when faced with an inhospitable environment. In response to adverse conditions, the fetus is able to modify its development to optimise its survival in the womb. This developmental plasticity of the fetus enables preservation of life in the short term, but may have unfavourable consequences for long-term health in postnatal life. During fetal life, the organs, vasculature and body systems undergo ‘critical periods’ of development. Disturbances to the environment during these sensitive periods can induce permanent changes in the structure, function and physiological set-points of organs and body systems. External environmental cues sensed by the mother and transduced to the fetus via the placenta, such as quality and quantity of nutrition and oxygen, together with internal cues arising from the mother such as hormones, nutrients and the function of the placenta, can induce developmental and functional adaptations in the fetus. Myriad epidemiological and experimental studies indicate that events occurring around the time of conception, through to pregnancy and early postnatal life, can sculpt the phenotype of the offspring.
Cardiovascular disease is one of the leading causes of death worldwide. Apart from genetic predisposition and traditional risk factors such as obesity, high blood pressure and smoking, it is now recognised that the quality of very early life is an important factor that can also infl uence cardiovascular disease risk. Research into the developmental origins of health and disease has demonstrated that factors such as the quality and quantity of maternal nutrition, hormonal status of the mother, stress, placental function and oxygen delivery to the fetus have implications for vascular structure and function and blood pressure in the offspring. The wealth of data emerging from experimental studies indicates that, more often than not, there are sex differences in the expression and severity of vascular dysfunction and blood pressure (Grigore et al. 2008). Female offspring tend to fare better than males in response to an early life insult. Furthermore, the raised blood pressure in females seen in some models is resolved after puberty. Whether there are differences in the ‘critical periods’ of development in utero or the ‘sensitivity’ to the insult for males and females has yet to be elucidated. However, sex hormones appear to modulate the expression of the cardiovascular phenotype developed in response to early life insults.
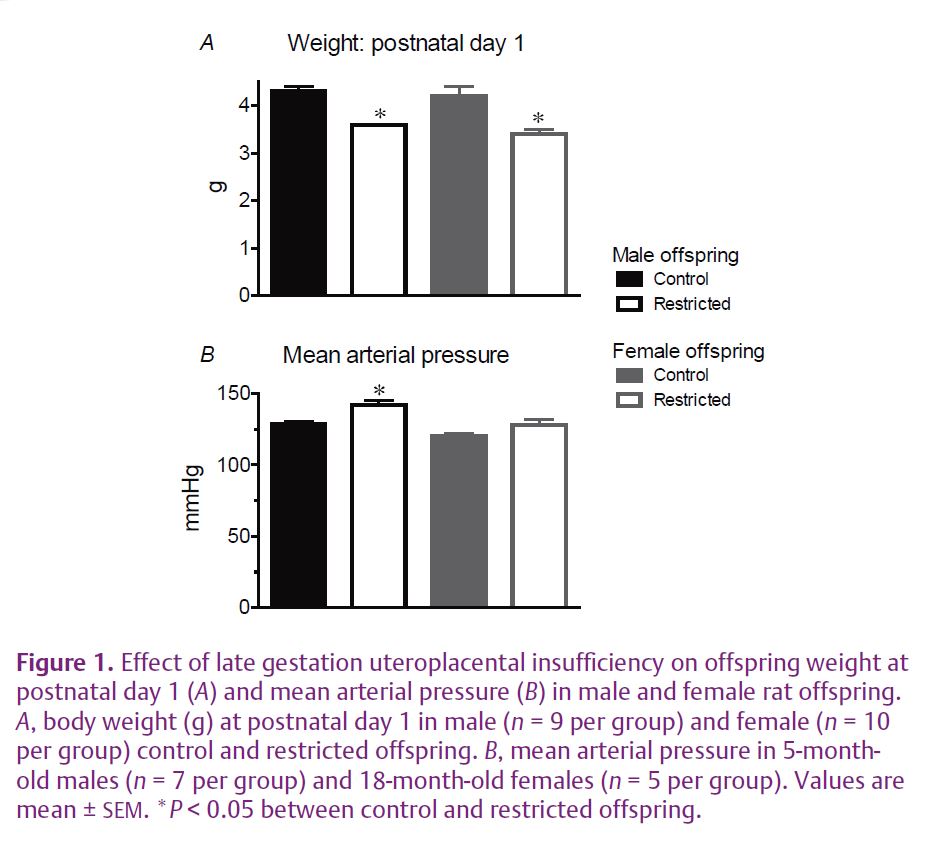
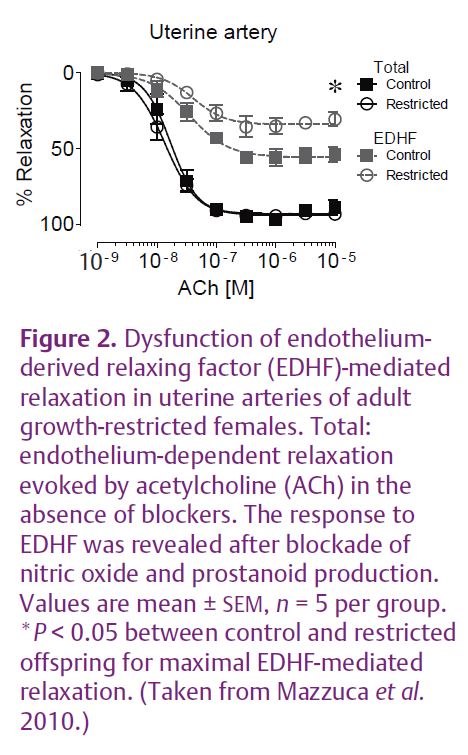
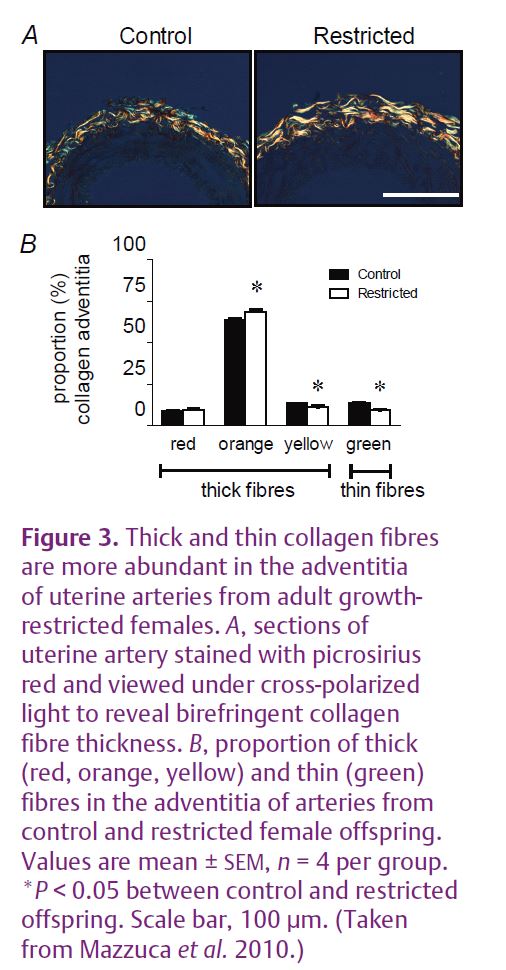
Uteroplacental insufficiency rather than maternal undernutrition is a common cause of intrauterine growth restriction in Western societies. Endothelial and smooth muscle function and arterial mechanical properties are important regulators of vascular function. Humans of low birth weight tend to have impaired endothelial vasodilator function and increased stiffening of the arteries, risk factors for cardiovascular disease (Norman, 2008). In our recent study we used a model of late gestation uteroplacental insufficiency by bilateral uterine artery and vein ligation in rats. This reduces the supply of nutrients and oxygen to the fetus. This model results in fetal growth restriction of 10–15% (Fig. 1A) without the confounding influence of maternal hypertension. We examined the effect on blood pressure and vascular function in adult female offspring. Previous studies of this model in our laboratory demonstrated that adult male growth-restricted offspring had raised blood pressure, reduced nephron endowment, reduced vasodilator capacity and increased arterial stiffness. The adult growth-restricted females also had a reduced nephron endowment, but were found to be normotensive (Fig. 1B) and had normal vascular function in the major resistance beds. The low nephron number alone was not sufficient to cause an elevation in blood pressure. However, closer investigation revealed that there was dysfunction in the uterine artery of growth-restricted females. Endothelial vasodilator function mediated by endothelium-derived hyperpolarizing factor (EDHF) was impaired (Fig. 2) (Mazzuca et al. 2010). EDHF is an important vasodilator mechanism in small arteries and arterioles. The stiffness of the uterine artery wall was also increased in growth-restricted females and this was associated with an increased proportion of thick, less compliant collagen fibres (Fig. 3). Importantly, vascular dysfunction was localised to the uterine artery and was not evident in the major resistance beds as in the males, and this probably contributes to the lack of hypertension in the females.
Upregulation of endothelial vasodilator factors in the uterine artery, including EDHF, is part of the natural adaptation that takes place in this vascular bed in response to pregnancy, to ensure adequate perfusion of the fetus. Inappropriate adaptation of the uterine vasculature in pregnancy is associated with compromised uteroplacental blood flow, growth restriction and pregnancy complications including maternal hypertension. Indeed, emerging evidence in the literature in humans and from our own laboratory studies in rats indicates that females born with low birth weights may have a higher risk of complications during pregnancy.
Research in the developmental origins of health and disease area has drawn attention to the critical role of early life environments in determining the cardiovascular and metabolic phenotype. It has also highlighted the complexity of the cardiovascular phenotype, and the reality that truly effective interventions in adults may need to be tailored according to the nature of the early life insult.
References
Bateson P (2001). Fetal experience and good adult design. Int J Epidem 30, 928–934.
Barker DJP (1995). Fetal origins of coronary heart disease. BMJ 311, 171–174.
Grigore D, Ojeda NB & Alexander BT (2008). Sex differences in the fetal programming of hypertension. Gend Med 5 (Suppl A), S121–S132.
McCance RA & Widdowson EM (1974). The determinants of growth and form. Proc R Soc Lond B Biol Sci 185, 1–17.
Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M (2010). Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol 588, 1997–2010. http://jp.physoc.org/content/588/11/1997.long
Norman M (2008). Low birth weight and the developing vascular tree: a systematic review. Acta Paediatr 97, 1165–1172.