
Physiology News Magazine
Targeted cell-based delivery of siRNA
Small interfering RNA (siRNA) is an ever more popular tool for silencing gene expression. But do experimenters always have to deliver siRNA directly into all the cells? Or can siRNAs pass from one cell to another via gap junctions? Ira Cohen and friends suggest that it all depends what connexins are around
Features
Targeted cell-based delivery of siRNA
Small interfering RNA (siRNA) is an ever more popular tool for silencing gene expression. But do experimenters always have to deliver siRNA directly into all the cells? Or can siRNAs pass from one cell to another via gap junctions? Ira Cohen and friends suggest that it all depends what connexins are around
Features
PR Brink, V Valiunas, S Doronin, I Potapova, RB Robinson,* MR Rosen,* RT Mathias, IS Cohen
Departments of Physiology and Biophysics at SUNY at Stony Brook, New York and *Department of Pharmacology, Columbia University, New York, USA
https://doi.org/10.36866/pn.63.16
The only direct form of intercellular communication in an ensemble of animal cells is provided by gap junction channels. All other forms of cell-to-cell communication must utilize the extracellular space. In vertebrates there are two unrelated families of proteins, connexins and pannexins, that form gap junction channels (Herve et al. 2005). In invertebrates another family of unrelated proteins, the innexins, serves the same function. Plant cells also communicate with each other; the structure they employ is called the plasmodesmata. Its pore diameter and length are both two orders of magnitude larger than that of any vertebrate gap junction channel and are known to allow passage of large solutes including siRNAs (Derrick et al. 1992; Staehelin, 1997; Vionnet, 2005).
In addition to passing ions to allow current flow, gap junction channels composed of connexins are permeable to a variety of larger molecules including nucleotides such as cAMP (Goldberg et al. 2004;Tsien & Wiengart, 1976). Although, Kolodny (1971) claimed that mRNA was permeable to gap junction channels his observations were compromised by a lack of controls for the actions of RNases. Hence, it was impossible to determine whether the molecules that moved from cell to cell were single nucleotides, multimers or combinations of both.
Because of the increasing therapeutic importance of siRNA, we decided to reinvestigate the permeability of gap junctions to short strands of nucleotides. We began by constructing tagged strands of nucleotides (morpholinos) of defined length and width. These oligonucleotides were resistant to the cells’ nucleases (Mudziak et al. 1996).
To determine experimentally if gap junctions are permeable to morpholinos we delivered a known concentration through a patch pipette to one cell of a pair in which junctional conductance and fluorescence intensity of the tagged oligonucleotide are simultaneously monitored over time (Valiunas et al. 2005). Figure 1A illustrates the transfer of a morpholino oligonucleotide 12 bases in length between two adult mesenchymal stem cells. Figure 1B depicts how a morpholino might gain access to a gap junction channel. We found that synthetic nucleotides like those depicted in Fig. 1 up to 24 bases in length traversed gap junction channels composed of connexin 43, but not those composed of connexins 32 and 26.
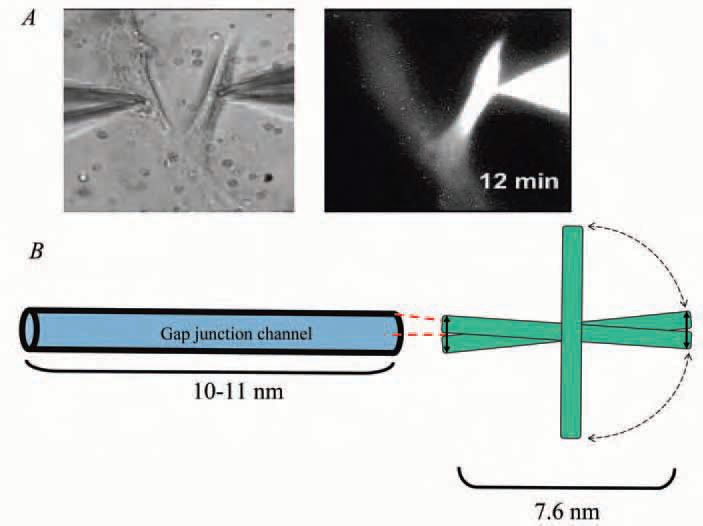
These studies with morpholino oligonucleotides demonstrate that the transfer of siRNA from cell to cell via gap junctions might be possible. They also suggest that the permeability could be connexin-specific. To test whether siRNA itself could cross gap junctions, we needed to identify an siRNA known to silence a specific gene. We chose one directed against polymerase β, a gene involved in DNA repair (Polosina et al. 2004). To investigate possible cell-to-cell transfer of the siRNA, three cell populations were transfected with a cDNA encoding polymerase β siRNA. Cell type 1 expressed connexin 43, cell type 2 expressed connexins 32 and 26, while cell type 3 was communication deficient (expressing no connexins). For each cell type we co-cultured wildtype cells not expressing siRNA with cells transfected to express the siRNA against polymerase β. The experimental protocol then called for separation of the two cell populations to determine if siRNA transfer resulted in a reduction of polymerase β message in the wildtype cells. We found that cells expressing connexin 43 were unique in transferring the siRNA from the transfected to the wild-type cells, resulting in knockdown of polymerase β message. These results confirmed what our studies with the synthetic oligonucleotides had already suggested: that gap junction channels composed of connexin 43 are sufficiently permeable to siRNA to facilitate silencing of a gene in an adjoining wild-type cell. The siRNA in this case was 22 bases long with dimensions of 1.1 by 7.4 nm and molecular weight of approximately 5kD.
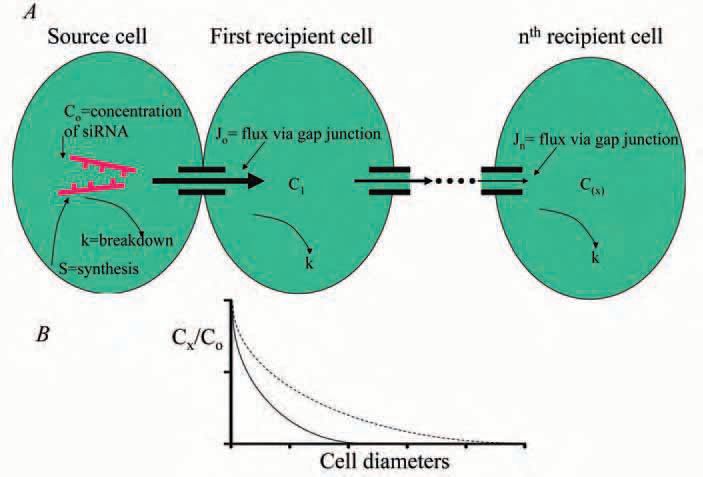
The permeability of gap junctions to siRNA demonstrates a new function, that of facilitating gene silencing from one cell to another. It suggests the potential for a source cell producing a siRNA, to affect gene expression within a syncytium of cells if an appropriate connexin is expressed. Yet, to understand how effective one cell might be in silencing gene activity in neighbouring cells we need to know how far the siRNA can move within a multicellular array. Junctional permeability is one of three important factors that can limit the spread of siRNA throughout a tissue. The other two are synthesis and degradation rates for the siRNA. Figure 2 is a simplified illustration of the role these factors play in the spread of siRNA within a tissue. Of interest is that siRNA from either an endogenous or an exogenous source survives and functions for many hours to days (Aliksy & Davidson, 2004) suggesting that siRNA movement from a source cell to those at distances of even a number of cell diameters away is possible.
siRNA is selective in silencing its gene target and as such presents an ideal therapeutic agent to silence the expression of proteins associated with various disease states in a variety of substrates. Yet to date its therapeutic application has been limited by an inability to deliver the siRNA selectively to the interiors of the target cells. Given our experimental results, the potential for siRNA to be delivered by one cell to a syncytium and affect gene expression in many other cells connected by gap junctions raises the possibility of using siRNA-loaded cells as the platform for a targeted delivery system. We have recently demonstrated that adult human mesenchymal stem cells express connexin 43 (Valiunas et al. 2004) and can deliver a pacemaker gene to the cardiac syncytium (Potapova et al. 2004). These same cells elicited no demonstrable rejection when maintained in canine heart for 6 weeks (Plotnikov et al. 2005), suggesting the possibility of their use from allogeneic sources.
In summary, the property of siRNA to permeate gap junction channels composed of connexins opens a door to targeted systemic silencing. How rapidly we avail ourselves of this opening and employ cell-based delivery for siRNA-based therapeutics remains to be seen.
References
Derrik PM, Barker H, Oparka KJ (1992). Increase in plasmodesmatal permeability during cell-to-cell spread of tobacco rattle virus from individually inoculated cells. Plant Cell 4, 1405-1412.
Goldberg G, Valiunas V & Brink PR (2004). Selectivity permeability of gap junction channels. Biochem Biophys Acta 662, 96-101.
Herve JC, Phelan P, Bruzzone R & White TW (2005). Connexins, innexins and pannexins: bridging the communication gap. Biochem Biophys Acta 1719, 3-5.
Kododny GM (1971). Evidence for transfer of macromolecular RNA between mammalian cells in culture. Exp Cell Res 65, 313–324.
Mudziak RM, Barofsky E, Barofsky DF, Weller DL, Huang SB & Weller DD (1996). Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nuc Acid Drug Dev 6, 267-272
Plotnikov AN, Shlapakova IN, Szabolcs MJ, Danilo PJ, Lu Z, Potapova I, Lorell BH, Brink PR, Robinson RB, Cohen IS & Rosen MR (2005). Adult human mesenchymal stem cells carrying HCN2 gene perform biological pacemaker function with no overt rejection for 6 weeks in canine heart. Circulation 11, 2II-221.
Polosina YY, Rosenquist TA, Grollman AP & Miller H (2004). Knockdown of DNA polymerase by RNA interference: recapitulation of null phenotype. DNA Repair 3: 1469 -1474.
Potapova I, Plotnikov A, Lu Z, Danilo P, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova I, Gao J, Pan Z, Herron A, Robinson RB, Brink PR, Rosen M & Cohen IS (2004). Human mesenchymal stem cells as a gene delivery system to fabricate a biological pacemaker. Circ Res 94, 952-959.
Staehelin AL (1997). The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 116, 11511165.
Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR & Cohen IS (2004) Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol 555, 617-626
Tsien, R.W & Weingart R (1976). Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol 260, 117142.
Valiunas V, Polosina Y, Miller H, Potapova I, Valiuniene L, Doronin S, Mathias RT, Robionson RB, Rosen, MR Cohen IS & Brink PR (2005). Connexin-specific cell to cell transfer of short interfering RNA by gap junctions. J Physiol 568, 459-468.
Voinnet O (2005). Non-cell autonomus RNA silencing. FEBS Letters 579, 5858-5871.
