
Physiology News Magazine
Taste receptors and glucose absorption in the small intestine
Natural sugars and artificial sweeteners activate taste receptors in the gastrointestinal tract to triple sugar absorption through the apical GLUT2 pathway and also to increase incretin secretion within minutes. George Kellett highlights the underlying mechanisms and looks at the dietary consequences and clinical opportunities
Features
Taste receptors and glucose absorption in the small intestine
Natural sugars and artificial sweeteners activate taste receptors in the gastrointestinal tract to triple sugar absorption through the apical GLUT2 pathway and also to increase incretin secretion within minutes. George Kellett highlights the underlying mechanisms and looks at the dietary consequences and clinical opportunities
Features
George Kellett
Department of Biology (Area 3), The University of York, York, UK
https://doi.org/10.36866/pn.71.34

Taste receptors and glucose sensing
Intestinal brush cells share morphological similarities with lingual taste cells and express the G protein α-gustducin, a key signalling protein in taste reception (Hofer et al. 1996). This discovery prompted a decade of speculation that nutrient sensing mechanisms may exist in intestine analogous to those for taste reception. Such speculation has finally become fact, prompted by the cloning of lingual sweet taste receptors.
The T1R family of taste receptors comprises three members, namely, T1R1, T1R2 and T1R3, which act as functional heterodimers. T1R1 + T1R3 in rodent act as a broad spectrum amino acid detector; in human they sense the umami or ‘delicious’ taste of monosodium glutamate in Chinese food. T1R2 + T1R3, on the other hand, detect sweet taste. Following cloning, transcripts for T1R2, T1R3, α-gustducin and also for members of the T2R family, which recognise bitter taste, were found in intestine. The hunt for a function was on.
Intestinal sugar absorption by SGLT1 and apical GLUT2
In the classical model, the Na+/glucose cotransporter SGLT1, a low capacity, low Km transporter, acts between meals as an effective scavenger by driving secondary active cotransport of glucose from the lumen (1–2 mM) uphill to the plasma (~ 4mM). Glucose is then transported into the blood by the high capacity, high Km facilitative transporter GLUT2, which is present normally only in the basolateral membrane of the absorptive cell (enterocyte).
However, SGLT1 becomes saturated at concentrations below those of 30-200 mM generated at the apical membrane after a meal. My lab therefore proposed the apical GLUT2 model (Kellett & Helliwell, 2000), in which SGLT1 exerts a key regulatory role by initiating rapid insertion of GLUT2 from intracellular vesicles into the apical membrane (Fig. 1). GLUT2 then provides the necessary additional transport capacity at high glucose concentrations; moreover, as glucose at the apical membrane increases, additional GLUT2 is inserted to cope with increased load. Finally, as glucose is absorbed, GLUT2 traffics rapidly away from the membrane.

Calcium and taste signals control apical GLUT2
During digestion, apical insertion of GLUT2 is dependent on a large, glucose-induced Ca2+ influx through the non-classical L-type channel Cav1.3 (Fig. 1), resulting from apical membrane depolarisation by SGLT1 (Mace et al., 2007b; Morgan et al. 2007). However, Ca2+ absorption is maximal at 20 mM glucose, but there is no increase in GLUT2 insertion. We therefore concluded there must be a second, downstream signal prompting GLUT2 insertion at higher glucose concentrations (>30 mM).
Taste receptors are activated by natural sugars at high concentrations, signalling through α-gustducin to activate PLC β2 and hence PKC βII, which promotes apical GLUT2 insertion. We noted that the sugar concentration range for activation coincided with that of 30-100 mM for apical GLUT2 insertion. In contrast, artificial sweeteners such as sucralose achieve a similar response at 1–2 mM. Sucralose is useful for intestinal studies, since it is neither absorbed nor metabolised and can only act through T1R2 + T1R3. We therefore quickly confirmed that taste receptors provide the second signal (Fig. 1) by showing that, in perfused rat jejunum in vivo, 1 mM sucralose doubled the rate of absorption of 20 mM glucose within minutes by increasing apical GLUT2 and associated absorption 3-fold (Mace et al. 2007a); on this time scale, SGLT1 was unaffected. Sucralose acts through the same PLC β2-dependent pathway as high glucose.
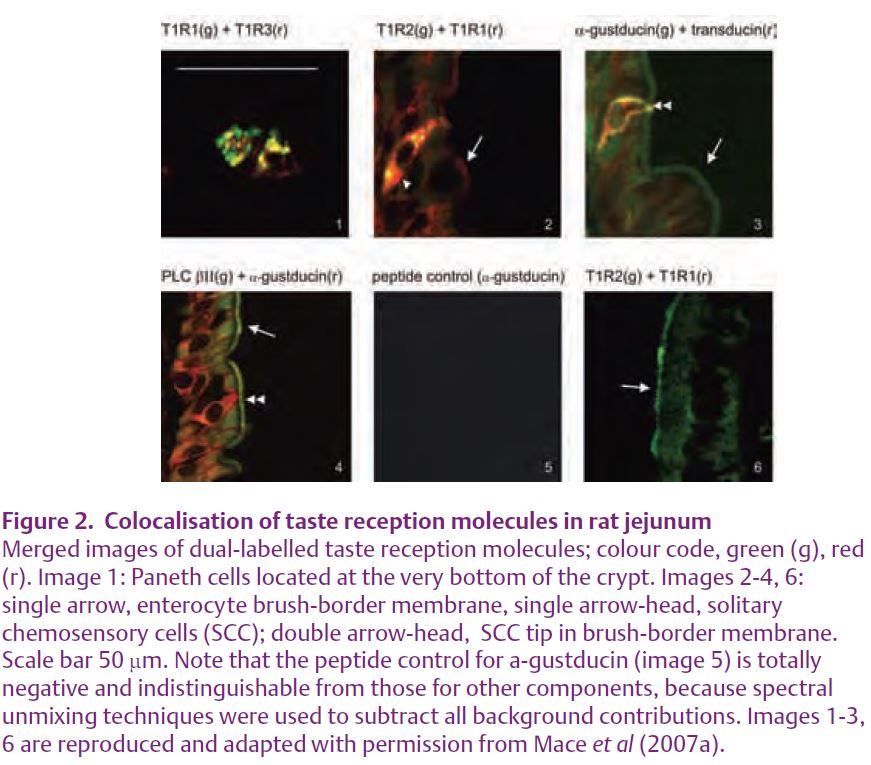
The presence of T1R1, 2 & 3, α-gustducin, transducin and PLC β2 throughout rat jejunum was demonstrated by Western blotting of apical membrane vesicles, which revealed that, like apical GLUT2, all taste reception components under-go rapid trafficking. As anticipated, immunocytochemistry demonstrated the presence of taste receptors, G-proteins and PLC β2 in SCC, used here as a general term to include brush, bipolar and entero-endocrine cells (Fig. 2). They were also found in Paneth cells and in jejunal enterocytes, which have all the necessary calcium and taste sensing machinery to account for increased glucose absorption (Fig. 1).
Margolskee and colleagues have since discovered that taste receptors in duodenal enteroendocrine cells can mediate glucose-induced secretion of incretins GIP (glucose-dependent insulinotropic peptide) and GLP-1 (glucagon-like peptide-1) from K and L cells respectively (Jang et al. 2007). Moreover, sucralose increases SGLT1 mRNA, protein and active glucose absorption of mice on a low carbohydrate diet for four weeks. Secretory and SGLT1 effects are both blocked in T1R3 or α-gustducin knockout mice (Margolskee et al. 2007). Nevertheless, a taste-sensing role for enterocytes is emphasised by the observation that fructose-induced increases in SGLT1 mRNA and protein are dependent on T1R3 in a clonal enterocytic Caco-2 human cell line, which expresses transcripts for T1R3 and α-gustducin and also T1R2 and T1R3 protein in the plasma membrane (Le Gall et al. 2007).
Diet, artificial sweeteners and health
The Western world is currently facing an epidemic of obesity and diabetes. It is generally helpful to reduce dietary calorie intake by the use of artificial non-caloric sweeteners; indeed, that is one reason why they are to be found worldwide in thousands of diet products, especially processed foods and fizzy drinks. However, artificial sweeteners bind to taste receptors synergistically with natural sugars to increase sugar absorption and incretin secretion within minutes. We therefore need to understand the long-term consequences for health of complex, but common dietary situations in which natural sugars and artificial sweeteners are consumed together, like eating hamburger and chips with a thirst-quenching fizzy diet drink. A reassessment of how artificial sweeteners can best be used to nutritional advantage may be necessary.
Equally, it might prove possible to use artificial sweeteners clinically to increase the secretion of incretins GIP and GLP-1 and improve insulin sensitivity. Such an approach would provide an alternative to the development of GLP-1 receptor agonists or GLP-1 analogues for the treatment of diabetes and obesity. Similarly, total parenteral nutrition (TPN) is often used in the management of infants suffering from malabsorption or other gastrointestinal disorders, because they cannot feed enterally. TPN, however, results in mucosal atrophy that can be ameliorated by supplementation with GLP-2 (Cottrell et al. 2006). Could artificial sweeteners provide a similar positive effect and enhance or restore the mechanism of absorption?
Conclusion
The discoveries outlined represent no more than a snapshot of a rapidly changing literature. Even so, they represent a sea change in our understanding of intestinal sugar absorption and invite exciting possibilities. Multiple, redundant regulatory pathways surely exist, as befits a single organ that mediates the input side of homeostasis. Understanding their integration and how one nutrient affects absorption of another will become fundamental areas of research. Moreover, clinical trials designed on the basis of new knowledge of apical GLUT2 and incretin secretion are required to assess the dietary impact of artificial sweeteners and to investigate new opportunities for beneficial dietary or pharmaceutical intervention. The
future of intestinal research is regulation.
Acknowledgments
The author thanks The Wellcome Trust for support.
References
Cottrell JJ, Stoll B, Buddington RK, Stephens JE, Cui L, Chang X & Burrin DG (2006). Glucagon-like peptide-2 protects against TPN-induced intestinal hexose malabsorption in enterally refed piglets. Am J Physiol Gastrointest Liver Physiol 290, G293-300.
Hofer D, Puschel B & Drenckhahn D (1996). Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A 93, 6631-6634.
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF & Egan JM (2007). Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A.
Kellett GL & Helliwell PA (2000). The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350 Pt 1, 155-162.
Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A & Brot-Laroche E (2007). Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol 213, 834-843.
Mace OJ, Affleck J, Patel N & Kellett GL (2007a). Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582, 379-392.
Mace OJ, Morgan EL, Affleck JA, Lister N & Kellett GL (2007b). Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol 580, 605-616.
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B & Shirazi-Beechey SP (2007). T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A.
Morgan EL, Mace OJ, Affleck J & Kellett GL (2007). Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol 580, 593-604.
