
Physiology News Magazine
The bustling world of hormone dynamics
Endocrinology Theme Lead update
Features
The bustling world of hormone dynamics
Endocrinology Theme Lead update
Features
Paul Le Tissier, University of Edinburgh, UK
Tim Wells, Cardiff University, UK
https://doi.org/10.36866/pn.118.44
It should go without saying that endocrinology is fundamental to physiology. Hormones are not only key mediators of homeostasis, but drive age-appropriate sea-changes in physiology within individuals. As such, they regulate and integrate all of the areas of physiology represented by the different Society themes. Recent advances in endocrinology have transformed the field. For example, identification of “new” hormones; realisation that well-established methodologies for quantification may be functionally misleading; and, identification of essential, previously unrecognised roles. These have provided dramatic new perspectives for endocrinologists. Rather than focus on these broader issues here, we would like to illustrate how these advances in endocrine research present challenges to our understanding, using examples from the growth hormone (GH) axis and its associated hormones.


The pattern of hormone secretion
Similar to many other hormones, it has long been recognised that GH secretion varies over multiple time scales, not only across the lifespan of an individual, but also with circadian and ultradian frequencies (Robinson and Hindmarsh, 2010; Steyn et al., 2016). The different ultradian pattern between males and females has important consequences for hormone action. Frequent sampling (e.g. every 10 mins for at least 12 hours) is required to fully characterise the pattern of pulsatile GH secretion, but in common laboratory animals, such as mice, this is only possible over a very limited time period (Steyn et al., 2011). New tools are now required to achieve temporal monitoring of hormone dynamics over these more extended periods. Use of nanomaterials and implanted devices utilising electrochemical or optical sensing of hormone levels or receptor activation holds promise for transforming the study of hormone dynamics, with the additional positive impact on the 3Rs – blood sampling no longer being necessary and longitudinal studies with animals acting as their own controls being possible.
Biologically active and inactive hormones
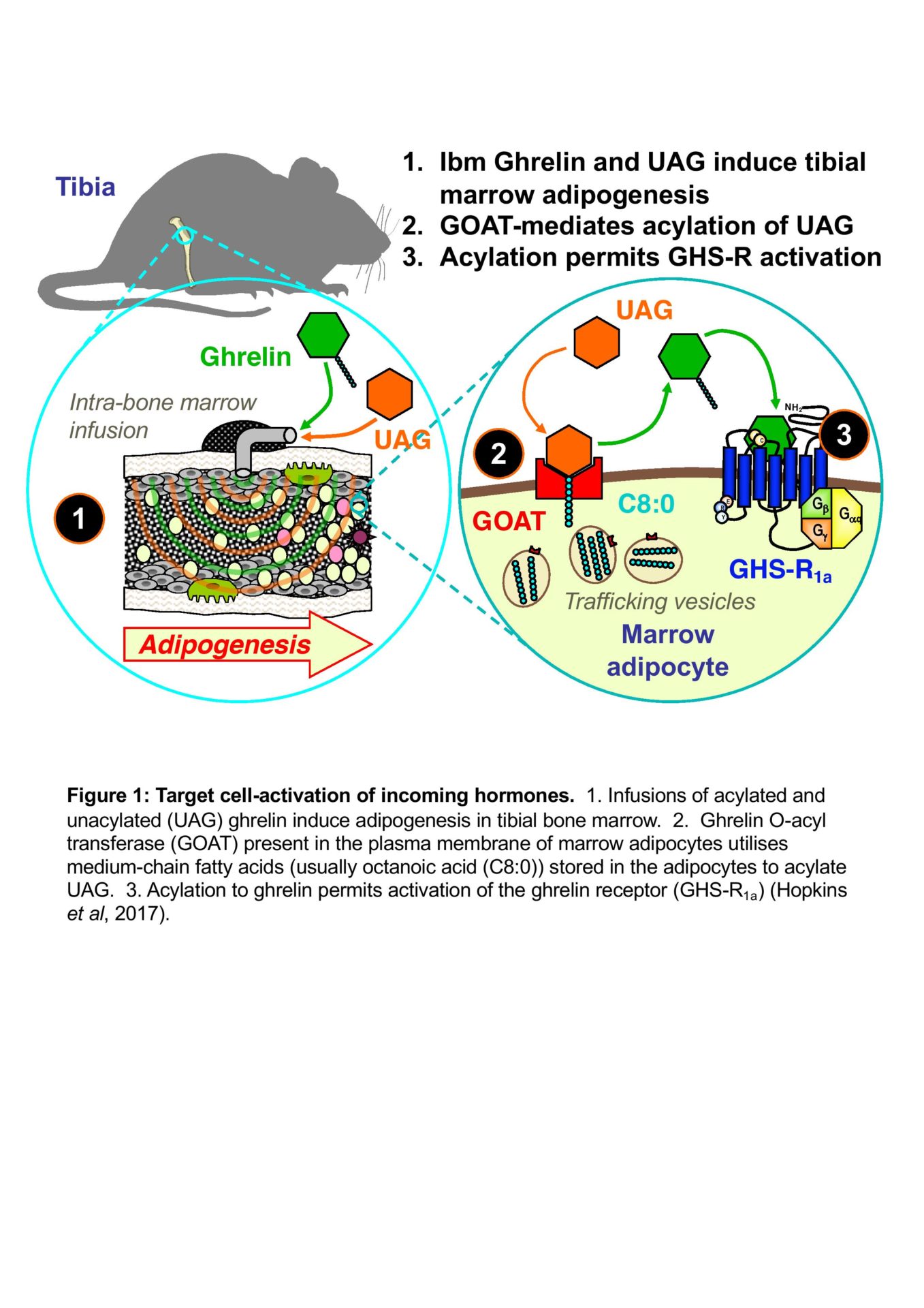
In common with many hormones, those of the GH axis circulate in both biologically active and inactive forms. This is exemplified by the gastric hormone ghrelin, which activates its receptor only when octanoylated, a reaction catalysed by ghrelin O-acyltransferase (GOAT) (Yang et al., 2008). The expression of GOAT in several tissues expressing the ghrelin receptor suggests that there may be local regulation of hormone activity in these tissues (Hopkins et al, 2017). This has far-reaching implications, as the ability of the target tissue to regulate the activation state of the incoming hormone (Fig. 1) could have a profound influence over physiological action and pharmacological targeting.
Axis cross-talk
The complexity of any endocrine axis results in endocrinologists commonly (and understandably) focusing on their specific hormone system of interest. However, the integration of physiological processes for survival and reproduction requires the co-ordinated modification of multiple endocrine axes and cross-talk between axes. As physiologists we need to understand how the “wood” functions as a whole and not just the individual “trees” within it. The relationship of the GH axis with stress is an excellent example of this: studies to date, many contradictory, indicate that stress affects the secretion of GH but the consequences can vary depending on species, time frame and severity (Steyn et al., 2016). This could also occur at multiple levels within the axis, with glucocorticoids, for example, both decreasing hypothalamic inhibition of GH secretion (Thakore et al., 1994) and increasing transcriptional activity of GH-secreting somatotropic cells (Thiell et al., 1993). The presence of several hormone receptors within a single cell type is, of course, one mechanism where integration of multiple axis functions can occur but there is increasing evidence for the modification of receptor responses by heterodimerisation. For example, the activity of melanocortin receptors, classically thought of as regulating stress and body weight have been shown to be modified by co-expression of the ghrelin receptor: the presence of both receptors in pituitary somatotropic cells may be a further mechanism integrating stress and the GH axes. Similar integration between the GH and gonadal axes may have important implications for current policy and health for those on reproductive steroid treatment.
Conclusion
These three features of the GH axis are common to many endocrine systems and indicate that continued study of individual hormones and their integrated interactions will continue to be a focus for many physiologists. Modulation of patterned secretion and hormone activation may underlie axis modification and interactions in response to altered physiological demand, such as pregnancy, as well as enhancing and preventing foetal exposure to circulating maternal hormones. Advances in these areas will require the adventurous combination of cross-disciplinary skills, from materials scientists, protein engineers, mathematicians and of course the endocrine physiologist to make sense of the wealth of data generated. Thus, although some may consider endocrinology to be a well-understood branch of physiology, a closer examination of factors such as hormone dynamics and local hormone activation, reveals a different picture. Understanding these more complex interactions will engender more informed policy development, more incisive diagnostic strategy and more appropriate patient treatment.
References
Hopkins AL et al. (2017). Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: evidence for target cell-induced acylation. Scientific Reports 7, 45541. DOI: 10.1038/srep45541
Robinson ICAF, Hindmarsh PC (2010). The Growth Hormone Secretory Pattern and Statural Growth. Comprehensive Physiology. John Wiley & Sons, Inc.. DOI: 10.1002/cphy.cp070512
Steyn FJ et al. (2011). Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 152(8), 3165 – 3171. DOI: 10.1210/en.2011-0253
Steyn FJ et al. (2016). Neuroendocrine Regulation of Growth Hormone Secretion. Comprehensive Physiology 6(2), 687 – 735. DOI: 10.1002/cphy.c150002
Thakore JH, Dinan TG (1994). Growth hormone secretion: the role of glucocorticoids. Life Sciences 55(14), 1083 – 1099. DOI: 10.1016/0024- 3205(94)00237-1
Theill LE, Karin M (1993). Transcriptional control of GH expression and anterior pituitary development. Endocrine Reviews 14(6), 670 – 689. DOI: 10.1210/edrv-14-6-670
Yang J et al. (2008). Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132(3), 387 – 396. DOI: 10.1016/j.cell.2008.01.017
