Physiology News Magazine
The ‘cost’ of breathing in heart failure
Heart failure patients develop a number of pulmonary abnormalities which increase the work and oxygen cost of breathing. Demands of the respiratory muscles are further accentuated during activities of daily living that require moderate exercise. In the face of limited cardiac reserve, heavily recruited respiratory muscles preferentially ‘steal’ blood flow from the working skeletal muscles. This phenomenon may be one mechanism that contributes to the enhanced perception of locomotor muscle fatigue commonly encountered in patients with heart failure.
Features
The ‘cost’ of breathing in heart failure
Heart failure patients develop a number of pulmonary abnormalities which increase the work and oxygen cost of breathing. Demands of the respiratory muscles are further accentuated during activities of daily living that require moderate exercise. In the face of limited cardiac reserve, heavily recruited respiratory muscles preferentially ‘steal’ blood flow from the working skeletal muscles. This phenomenon may be one mechanism that contributes to the enhanced perception of locomotor muscle fatigue commonly encountered in patients with heart failure.
Features
Thomas P. Olson and Bruce D. Johnson
Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN 55905, USA
https://doi.org/10.36866/pn.82.42


The two most common symptoms heart failure (HF) patients suffer from include shortness of breath (dyspnoea) and fatigue. As such, exercise intolerance is a hallmark of symptomatic HF. Initially, investigators sought to determine which measure of cardiac function (left ventricular ejection fraction, cardiac output, filling volumes, etc.) best related to an individual’s exercise tolerance. Unfortunately, these studies failed to find a clear link between resting measures of cardiac function and exercise tolerance in patients with HF.
While altered cardiac function clearly plays an initiating role, HF becomes a systemic illness which impacts on multiple organ systems. One system particularly influenced is the pulmonary system. This system is intimately linked with the cardiovascular system both anatomically and haemodynamically in that the lungs lie in series with the heart, share a common surface area within the thoracic cavity, and are exposed to similar intrathoracic pressure changes. Heart failure patients often develop mild pulmonary function impairments including restrictive and to a lesser extent obstructive changes, reduced lung diffusing capacity (surface area for gas exchange), and reduced respiratory muscle strength. They also require greater levels of ventilation for a given metabolic demand, due to a more rapid–shallow breathing pattern, altered matching of ventilation to perfusion, and chronic, mild hyperventilation. The end result of these pulmonary manifestations of HF is an elevated work and cost of breathing, particularly during activities of daily living that require moderate exercise intensities (Olson et al. 2006). This increase in the work and cost of breathing during activity is particularly concerning when coupled with a severely blunted ability to increase cardiac output due to the disease pathology.
Exercise elicits an increased need for blood flow to both the respiratory and working skeletal muscles. Under normal circumstances, during exercise in healthy individuals, a combination of localized vasoconstriction (renal, splanchnic, etc.) and an increase in cardiac output combine to meet the demands of both the respiratory and locomotor muscles. This appears to be the case up to approximately 75–80% of maximal intensities. However, at higher intensities, the respiratory muscles begin to preferentially recruit blood flow at the expense of the working skeletal muscle. In fact, Harms and colleagues suggested, during maximal cycle exercise in healthy individuals, reducing respiratory muscle work by ~60-65% resulted in ~4–5% increase in blood flow to the legs whereas increasing respiratory muscle work by ~25–30% led to ~7% reduction of blood flow to the legs (Harms et al. 1997). From these findings, the authors concluded that the work of breathing exhibited during heavy exercise results in a reflex vasoconstriction of the locomotor muscle vascular beds contributing to reduced leg blood flow.
As noted previously, due to a number of pulmonary system manifestations from the failing heart, the work and oxygen cost of breathing for a given level of oxygen consumption will be accentuated in HF and requires greater relative blood flow than that seen in healthy individuals. This increase becomes particularly exacerbated during increasing intensities of exercise; however, it is possible that even low levels of physical activity in HF patients (e.g. representing typical activities of daily living) may result in a redistribution of blood flow away from the working skeletal muscles to the respiratory muscles in an effort to compensate for the respiratory muscle work in the setting of limited cardiac reserve. If true, a respiratory muscle blood flow ‘steal’ from the locomotor muscles may be one mechanism which contributes to heightened perceptions of fatigue that are prevalent in this population. Consistent with this premise, previous work in animal models of HF have suggested that the diaphragm (a primary muscle of respiration) will take blood flow from working skeletal muscles during physical activity. In addition, O’Donnell and colleagues have shown that providing human HF patients with inspiratory pressure support using a ventilator resulted in longer submaximal steady-state exercise duration and reduced leg discomfort (O’Donnell et al. 1999). Further, Borghi-Silva et al. (2008) have demonstrated that HF patients undergoing constant load exercise at 70–80% of peak work while using a proportional assist ventilator increased skeletal muscle oxygenation and an estimate of leg blood volume.
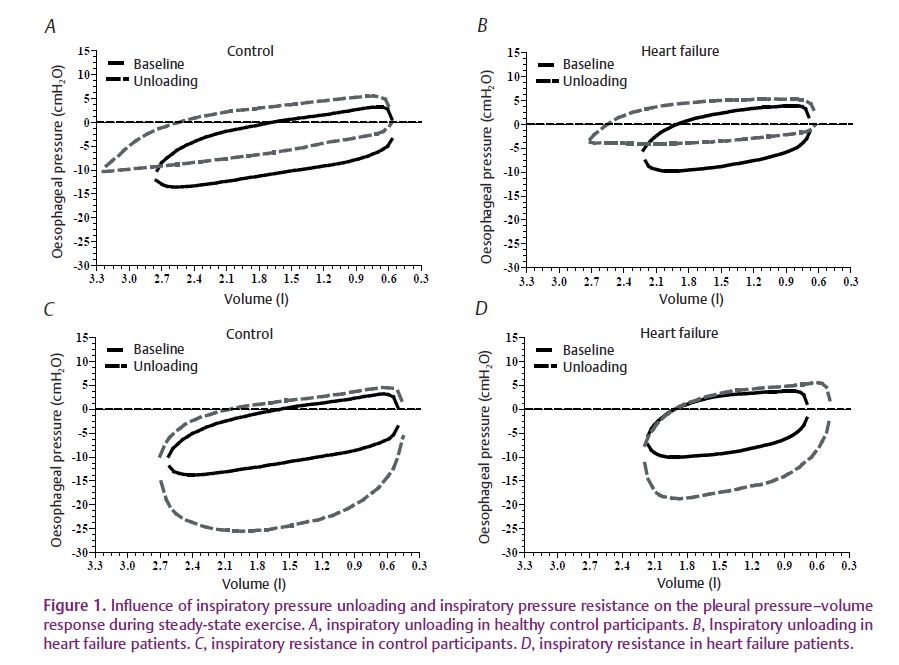
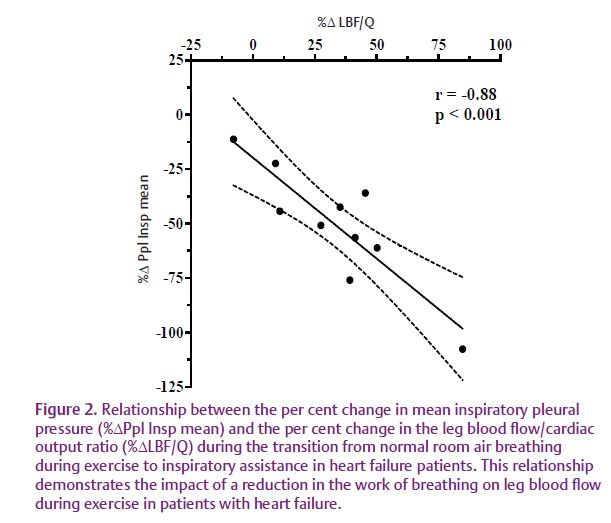
Accordingly, we sought to determine the relationship between respiratory muscle work and leg blood flow during moderate-intensity exercise in patients with HF (Olson et al. 2010). During this study, HF patients exercised at a constant submaximal rate of 60% peak work while breathing room air, with inspiratory muscle unloading via a ventilator, or with inspiratory muscle resistance (Fig. 1). During these exercise bouts we measured the work of breathing using an oesophageal balloon to quantify intrathoracic pressure (a measure of work) as well as leg blood flow by thermodilution using catheters in the femoral vein. We observed that reducing the normal inspiratory muscle work via inspiratory pressure support during exercise resulted in a significant increase in leg blood flow in HF patients, but not in healthy control participants (Fig. 2). In contrast, increasing the work of breathing by adding inspiratory resistance did not alter leg blood flow or cardiac output in HF patients, but increased cardiac output in the healthy controls. These results suggest that the HF patients were able to redistribute blood flow to the locomotor muscles when respiratory muscle work was reduced, but were unable to reduce locomotor blood flow beyond that observed during normal breathing when inspiratory muscle work was increased. This was most probably due to the lack of cardiac reserve and a maximal level of vasoconstriction in the active locomotor muscles.
In summary, our research in HF is consistent with that observed in healthy athletic adults and is an example of the intricate balance between the demands for blood flow relative to the available reserves. For the HF patient, very modest amounts of activity appear to challenge the ability to deliver blood flow to both the respiratory muscles and locomotor muscles, while in the athlete this only occurs near maximal levels of exercise. However, in both cases, the respiratory muscles appear to preferentially ‘steal’ blood flow at the expense of the locomotor muscles. This ‘steal’ phenomenon may result in a mismatch in oxygen supply and demand and be one mechanism which contributes to the enhanced perception of fatigue commonly encountered by patients living with HF.
References
Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaca D, Lira-Filho EB, Ribeiro D, Nery LE & Neder JA (2008). Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol 294, H2465–H2472.
Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573–1583.
O’Donnell D, D’Arsigny C, Raj S, Abdollah H & Webb K (1999). Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med 160, 1804–1811.
Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB & Johnson BD (2010). Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588, 2487–2501. http://jp.physoc.org/content/588/13/2487.long
Olson TP, Snyder EM & Johnson BD (2006). Exercise disordered breathing in chronic heart failure. Exerc Sport Sci Rev 34, 194–201.
Acknowledgements
Dr Olson’s research program is supported by NIH/NCRR CTSA–KL2 RR024151. Dr Johnson’s research program is supported by NIH 5R01HL71478-7.