Physiology News Magazine
The embryo and a lifetime of staying healthy
Can the risk of adult-onset disease be influenced more by your development as an embryo than your lifestyle as an adult?
Features
The embryo and a lifetime of staying healthy
Can the risk of adult-onset disease be influenced more by your development as an embryo than your lifestyle as an adult?
Features
Adam J Watkins & Tom P Fleming
School of Biological Sciences,University of Southampton, UK
https://doi.org/10.36866/pn.72.25

The development of adult-onset diseases, such as type II diabetes, obesity and cardiovascular disease, has traditionally been associated with adult behaviour such as a sedentary lifestyle, poor diet and smoking.
However, over recent years a growing body of evidence from both human and animal models has challenged this concept and reveals that environmental conditions experienced during critical windows of gestation can have an even greater influence on adult health (Barker & Bagby, 2005; Hanson & Gluckman, 2008). Indeed, research from human, rodent and livestock studies has identified the earliest stages of development, prior to embryo implantation, to be particularly sensitive to changes in their immediate environment either in vitro (e.g. culture conditions) or in vivo (e.g. maternal diet) with long-term consequences into adult life (Watkins et al. 2007, 2008a, b, c).

Mammalian preimplantation development encompasses the period between fertilisation of the egg and implantation into the uterine wall (approximately 4 days in the mouse and 6 in the human; Fig. 1). In vivo, the preimplantation embryo is largely dependent upon the maternal reproductive tract environment to provide the necessary metabolites and growth factors to sustain and progress development. This dependency can be easily demonstrated by removing the embryo from the reproductive tract and culturing it in vitro. This commonly results in a slower rate of development and a reduced number of embryonic cells. However, the effects of embryo culture are not solely confined to the period of preimplantation development but can persist into later pregnancy and the postnatal period affecting growth, physiology and health of the offspring. One of the most dramatic examples of how embryo culture conditions can impact upon long-term development is the phenomenon of ‘large offspring syndrome’ (LOS). LOS is observed following the culture of sheep and cattle embryos, usually in the presence of serum. As the name suggests, LOS animals are born significantly heavier with abnormal sizes of internal organs, altered patterns of gene expression and increased rates of perinatal death (Sinclair et al. 2000). In mice, embryo culture has been associated with altered gene expression and regulation, the development of postnatal hypertension, altered renin-angiotensin system (RAS) homeostasis, and abnormal behaviour and memory characteristics (Fig. 2; Watkins et al. 2007, 2008a). These findings from animal studies pose questions about the long-term health of children conceived via assisted reproductive techniques (ART). In humans, the impact of ART on long-term health is, at present, difficult to gauge. Whilst children conceived through ART are more likely to be born prematurely and with a low birth weight (<2500g), this has been attributed to the increased incidence of ART-induced multiple pregnancies and inherent defects in the quality of parental sperm/eggs (Ludwig et al. 2006). It is therefore essential for follow up studies to be continued to assess the safety of ART.
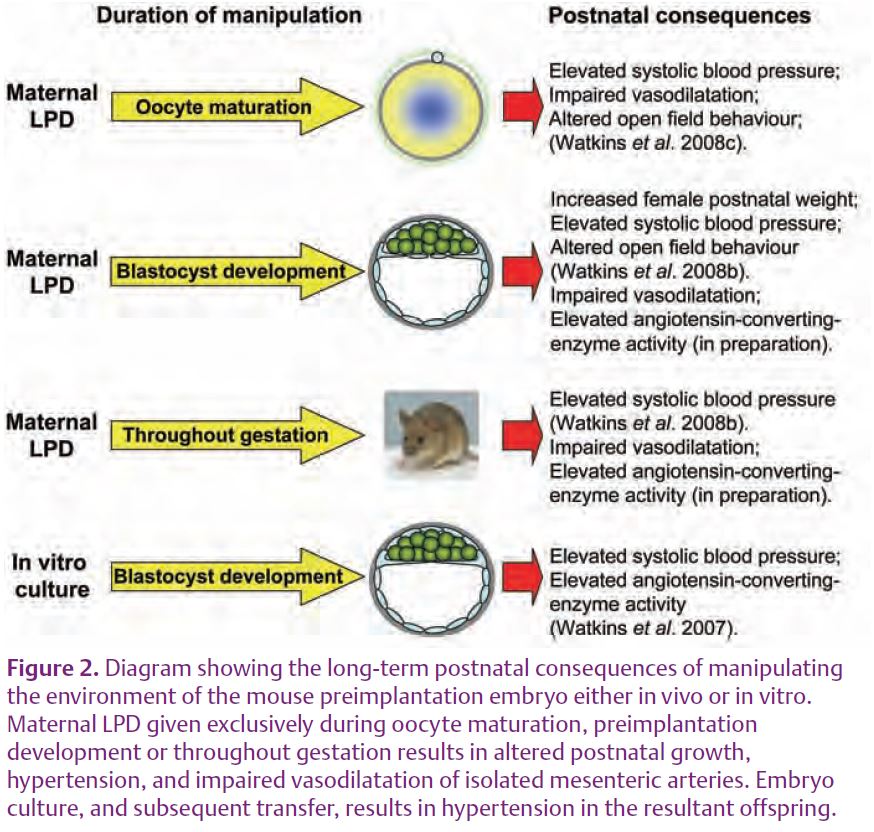
Manipulating maternal diet has allowed us to determine the long-term consequences of altering the environment of the preimplantation embryo in vivo. When we provided a low protein diet (LPD) to mothers exclusively during the preimplant-ation period (Emb-LPD) the female offspring were born significantly heavier than control offspring. These females remained heavier for up to 6 months of age and displayed abnormal locomotor activity in the open field test (Fig.2; Watkins et al. 2008b). Moreover, both male and female offspring from the maternal LPD treatment became hypertensive throughout adult life (Watkins, 2008b). Similarly, we found that in the rat, maternal LPD during the same period of development reduced the number of cells present within blastocysts and altered the pattern of gene expression in the blastocyst and later conceptus stages, leading to gender-specific changes in postnatal growth and systolic blood pressure (Kwong et al. 2000, 2006, 2007).
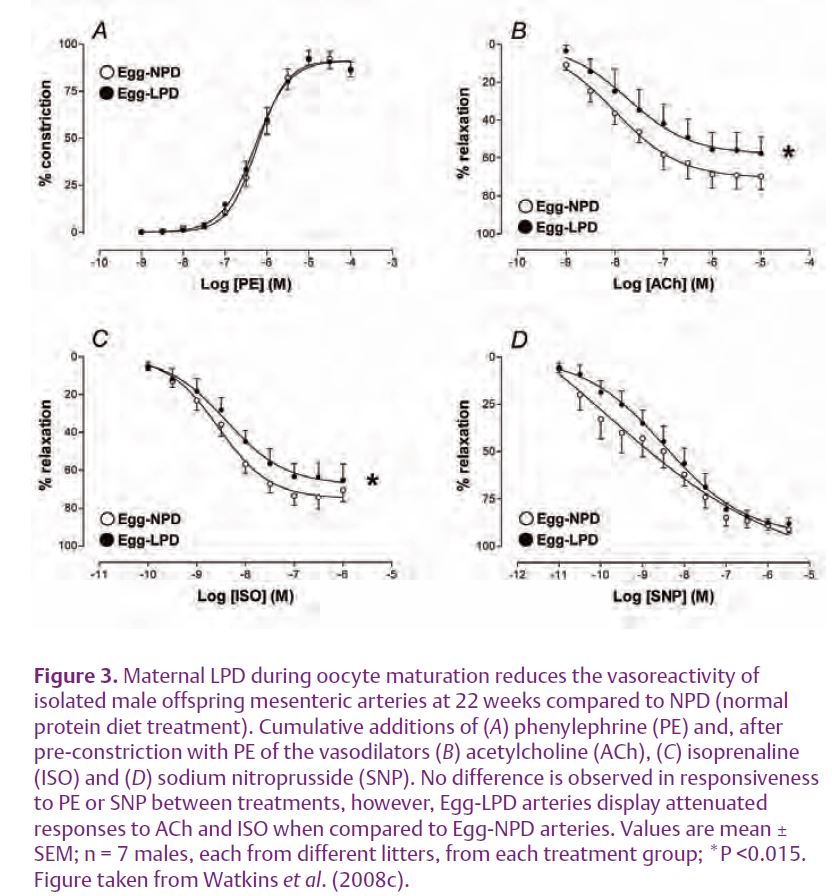
In sheep, maternal undernutrition prior to conception, or the feeding of a high protein diet, reduces the viability of oocytes and embryos, alters the patterns of fetal and postnatal growth and fetal endocrinology status, induces hypertension and increases the responsiveness of arteries to vaso-constrictive compounds (Cleal et al. 2007; Sinclair et al. 2007; Watkins et al. 2008a). Recently, we observed that maternal LPD exclusively during mouse oocyte maturation (3.5 days prior to mating; Egg-LPD) resulted in offspring developing hypertension and altered patterns of open field behaviour (Fig.2; Watkins et al. 2008c). This hypertensive state was associated with significant impair-ment in mesenteric artery vasodila-tation in response to the endothelial-dependent and independent vasodilators acetylcholine (ACh) and isoprenaline (ISO) respectively when compared to controls (Fig. 3).
The global prevalence of diseases such as obesity, cardiovascular disease and diabetes is increasing at a dramatic rate and is no longer confined to developed countries. The studies outlined above demonstrate a clear link between egg and embryo environment and development and the onset of these adult diseases in animal models. Further studies are urgently required to determine the molecular, biochemical and epigenetic mechanisms underlying such associations, and to the extent to which these relationships may exist in humans.
Acknowledgements
Our work has been supported by the National Institutes of Health, USA as part of the NICHD National Cooperative Program on Female Health and Egg Quality under cooperative agreement U01 HD044635, the Gerald Kerkut Charitable Trust and the Medical Research Council.
References
Barker DJ & Bagby SP (2005). Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol 16, 2537–2544.
Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, Hanson MA, & Green LR (2007). Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci U S A 104, 9529–9533.
Hanson MA & Gluckman PD (2008). Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol 102, 90–93.
Kwong WY, Wild AE, Roberts P, Willis AC, & Fleming TP (2000). Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 127, 4195–4202.
Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, & Fleming TP (2006). Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 132, 265–277.
Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, & Fleming TP (2007). Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev 74, 48–56.
Ludwig AK, Sutcliffe AG, Diedrich K, & Ludwig M (2006). Post-neonatal health and development of children born after assisted reproduction: a systematic review of controlled studies. Eur J Obstet Gynecol Reprod Biol 127, 3–25.
Sinclair KD, Young LE, Wilmut I, & McEvoy TG (2000). In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod 15 Suppl 5, 68–86.
Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, Lea RG, Craigon J, McEvoy TG, & Young LE (2007). DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A 104, 19351–19356.
Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, Osmond C, Hanson M, & Fleming TP (2007). Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A 104, 5449–5454.
Watkins AJ, Papenbrock T, & Fleming TP (2008a). The preimplantation embryo: handle with care. Semin Reprod Med 26, 175–185.
Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, Eckert JJ, Wild AE, Hanson MA, Osmond C, & Fleming TP (2008b). Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 78, 299–306.
Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, & Fleming TP (2008c). Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol 586, 2231–2244.