
Physiology News Magazine
The impact of nociception and pain: Implications for animal welfare legislation
Features
The impact of nociception and pain: Implications for animal welfare legislation
Features

https://doi.org/10.36866/pn.126.12
Dr Lynne U Sneddon
University of Gothenburg, Sweden
Nociception (from the Latin noce-re “to harm”) is the simple detection of noxious, potentially damaging stimuli and is usually accompanied by a reflex withdrawal response away from the danger. Receptors for nociception, termed nociceptors, are the subject of intense study, from their anatomy and molecular biology through to their electrophysiological properties (Sneddon, 2018). These neurons convey information about tissue-damaging stimuli to the central nervous system and alert the animal to the risk of injury. In effect, they act as an alarm system and elicit the adaptive response of retreating from the offending stimulus. Nociceptors also underlie the sensation and affective dimension of pain where tissue damage leads to feelings of discomfort and suffering.
The International Association of the Study of Pain (IASP), a clinical society, recently changed their definition of pain from “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Merskey, 1979) to “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja et al., 2020). This may appear as a slight change to the terminology, but it is an incredibly important one as it recognises that pain may occur in other animals and not just humans. Previously, criticisms of the original definition included the concept that pain could only be felt by humans who could self-report their pain and thus excluded infants, adult humans with medical conditions that make communication difficult or impossible, and of course animals who we do not share common language with.
This is a dramatic shift in the dogma surrounding pain and this definition accepts that pain is not just a human experience. Of course, animal models of nociception and pain, which have yielded so many important insights into our fundamental knowledge of human pain, must experience some form of pain to be relevant otherwise why study them? In humans, self-reporting of the pain experience is the gold standard; however, with humans and animals that cannot communicate using spoken language, how do we tell if they are in pain? The primary way in which pain is assessed is in behavioural responses and so under the new IASP definition if an individual is displaying behavioural and physiological changes that resemble pain then it should be accepted that pain is occurring. So, it is the insertion of this word “resembling” that creates a paradigm shift in the doctrine surrounding pain and acknowledgement of its existence in other animals.
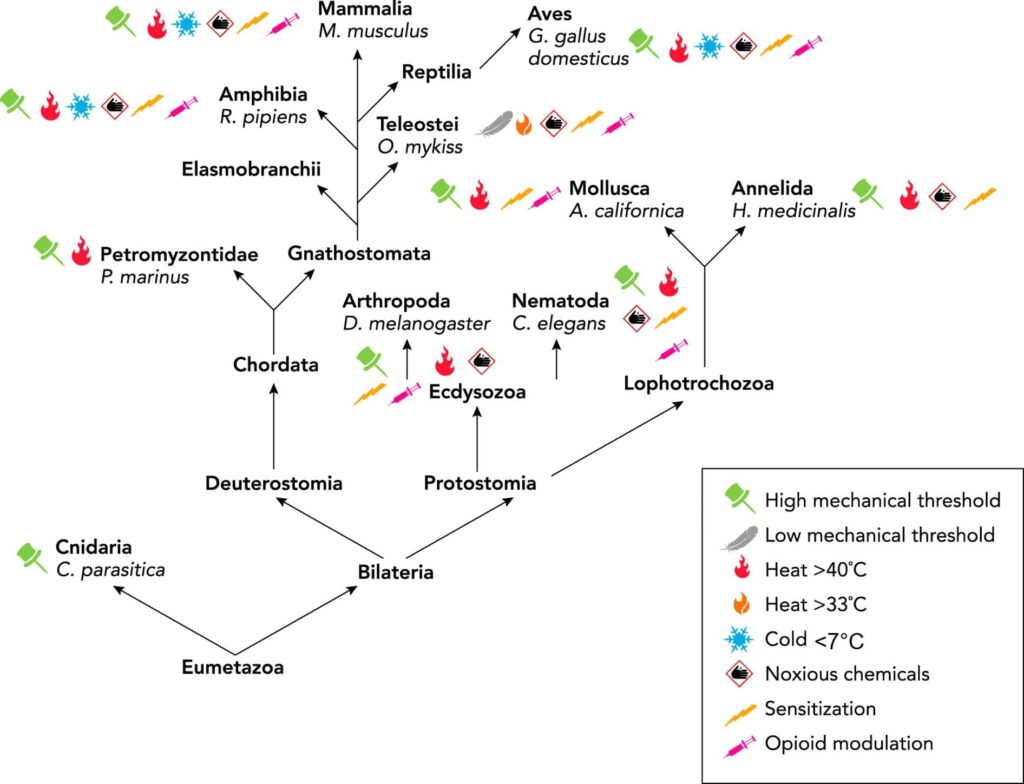
Why study pain in animals?
The use of animals in nociception and pain studies has led to dramatic discoveries and has really fuelled our understanding of the basic biology of this important sensory system. Pain can be debilitating and reduce the quality of human life and this also poses challenges for the welfare of animals.
We must ask ourselves, why have animals become such important experimental models for the study of clinical pain? Using animal models with perhaps a less complex nervous system (e.g. invertebrates) may not be immediately intuitive, but their study can yield insights into the mechanisms of nociception and pain that lead to the discovery of new therapeutics. Their less complex structure can allow much more detailed analysis of how the nervous system works using genetic, physiological and molecular tools and, further, they are considered as Replacement under the 3Rs umbrella and are believed to be a more ethical choice to replace vertebrates without the requirement for acquiring experimental licensing or governmental permits. Studying animals can also help us understand the comparative aspects as well as the evolutionary conservation of nociception and pain. Finally, many laws and guidelines demand we keep animals in a painfree state. So by studying the occurrence of pain from nociceptors through to whole-animal responses we can inform pain management and seek to avoid, minimise, or alleviate pain in captive contexts such as the laboratory or in farming.
Invertebrate models of nociception, where neuronal activity can easily be linked to behaviour, include the sea slug (Aplysia californica), roundworm (Caenorhabditis elegans), fruit fly (Drosophila melanogaster) and the leech (Hirudo medicinalis) and so on. These have advantages over rodent models due to the lack of need for ethical permission and due to easier monitoring of their nervous system. Much of the underlying molecular biology and physiology of nociceptors is strikingly similar to that of mammals, including opioid modulation (Fig.1; Sneddon, 2018; 2019), and this has led to the identification of new targets for the alleviation of pain (e.g. analgesics). For example, a chronic pain model in Drosophila has demonstrated that the Bone Morphogenetic Protein (BMP) pathway downstream of the hedgehog (Hh) signalling pathway, known to be involved in nociceptive sensitisation in response to injury, has been thoroughly dissected and yielded new insights into the mechanism of chronic pain. In a recent study, the proteins Brinker (Brk), and Schnurri (Shn) were suppressed in nociceptors using an RNA-interference (RNAi) knockdown approach in Drosophila. Knockdown of Brk resulted in hypersensitivity in the absence of injury, suggesting a role in suppressing nociceptive sensitivity. Whereas when Shn was knocked down in the Drosophila nociceptors, allodynia (pain response to a stimulus that does not normally provoke pain) was not seen after injury, showing that this transcriptional activator is involved in promoting hypersensitivity after injury (McParland et al., 2021). Both Brk and Shn can now be considered as novel targets in chronic pain treatment in humans and other animals. So, do fruit flies experience chronic pain given these results? If so, should we safeguard their welfare? This is certainly becoming a growing concern amongst scientists and the public (Delvendahl et al., 2022).
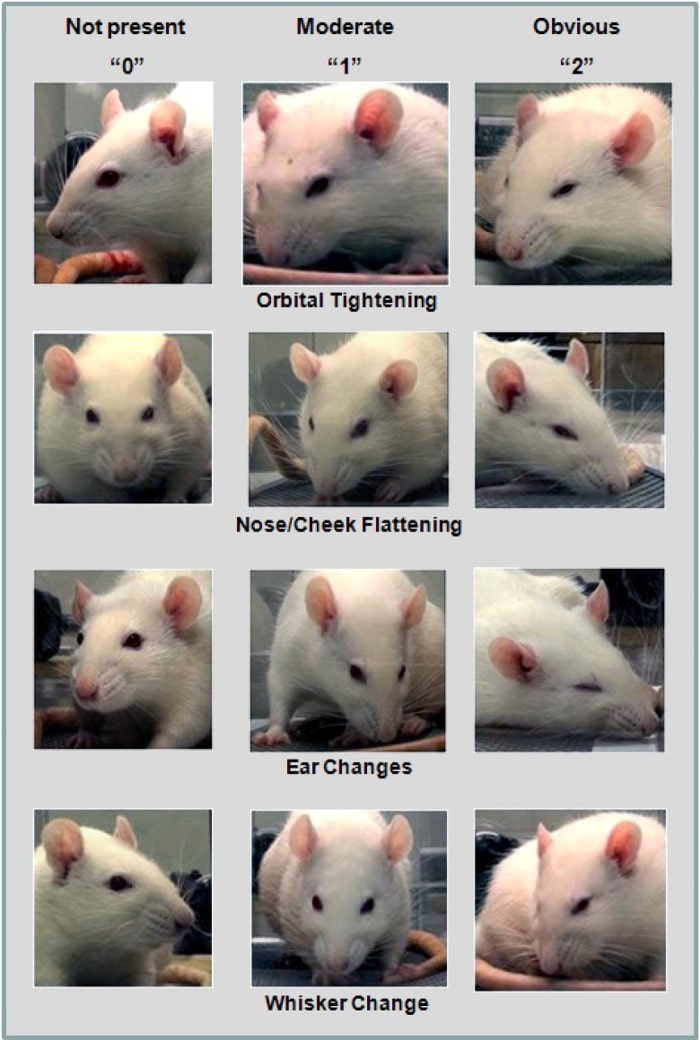
1. Orbital Tightening: Rats in pain display a narrowing of the orbital area, manifesting either as (partial or complete) eye closure or eye “squeezing.” 2. Nose/Cheek Flattening: Rats in pain display successively less bulging of the nose and cheek (see above), with eventual absence of the crease between the cheek and whisker pads. 3. Ear Changes: The ears of rats in pain tend to fold, curl and angle forwards or outwards, resulting in a pointed shape. The space between the ears may appear wider. 4. Whisker Change: The whiskers of rats in pain move forward (away from the face) from the baseline position, and tend to bunch, giving the appearance of whiskers standing on end (Reproduced from Sotocina et al., 2011 under the terms of the CC BY 3.0).
Why is pain so important in animal welfare legislation?
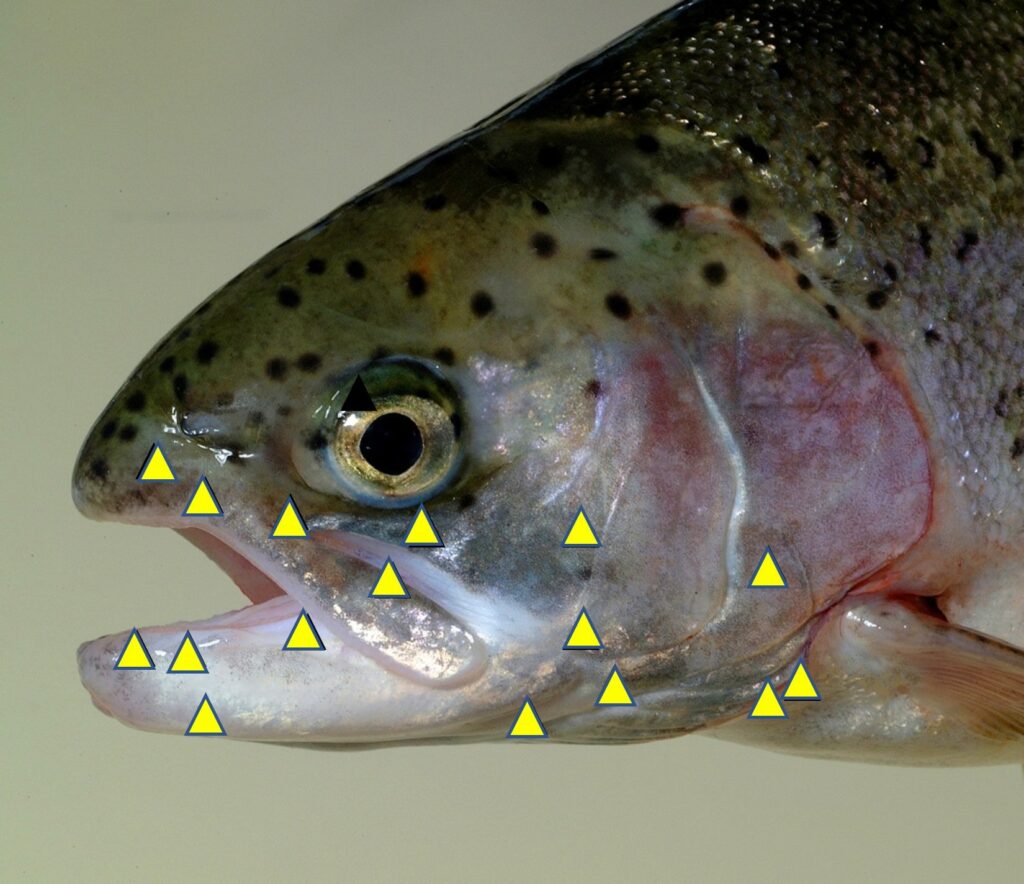
When deciding which animals to protect under legislation and guidelines, pain is the key factor. Indeed even in 1789, the philosopher Jeremy Bentham provided a famous quote: “the question is not, Can they reason? nor, Can they talk? but, Can they suffer?” Providing sound scientific evidence that an animal is capable of experiencing pain and the associated suffering and discomfort makes a compelling case for their protection. The scientific legislation concerning the use of adult vertebrate animals and cephalopods in the EU and the UK states that pain should be avoided or minimised to reduce suffering in laboratory animals. However, this is often not applied equally to different species. For example, pain relief protocols are routinely used and the assessment of pain is well developed in rodents (see Fig.2 for rat grimace scale, Sotocina et al., 2011; mouse and rabbit grimace scales can be found on the NC3Rs UK website). This is not the case for fish models where the assessment of pain is less well studied and analgesic drugs are rarely used or reported. In some regions there are no welfare laws pertaining to fish and only local ethical review occurs in experimentation. With the first publication of nociceptors in fish 20 years ago (Fig.3; Sneddon, 2002), the situation has been transformed and the treatment of fish has been enhanced through a better understanding of the biology of pain in these animals (Sneddon, 2019). Thus far we know fish nociceptors are comparable with mammals because the underlying molecular biology is similar and behavioural and physiological responses to painful treatment are prevented by analgesic drugs. By studying neuroanatomy, electrophysiology, stress physiology and behavioural responses to pain, it is now largely accepted that fish experience pain. This research has informed changes in attitudes not only in science but in other fields. In aquaculture, a recent report adopted by the European Union states “There is growing evidence that fish, like other vertebrate animals, are sentient beings, and that they can experience pain and distress” (SCAR, 2018).
Pain in cephalopods and decapod crustaceans
Headlines from many media outlets were emblazoned with the outcome of a UK government-funded report on the existence of pain in cephalopods and decapod crustaceans. Currently adult cephalopods such as octopus, squid, cuttlefish and Nautilus are included in the EU and UK science legislation (e.g. laboratory animals) but not in the UK Animal Welfare Act, which pertains to animals held in captivity (e.g. companion and production animals) but not to wild animals. Decapods such as crab, lobsters, prawns and shrimps were not included in the European science legislation in 2010 as there was not enough evidence for pain in these animals at that time. This new report on these decapods and cephalopods reviews the scientific evidence for nociception and pain in these animal groups and comes to the conclusion that they should be afforded protection under UK law.
In decapods, there is behavioural evidence that resembles pain (review in Elwood, 2019). Application of noxious chemicals to antennae in prawns results in rubbing and this is reduced by morphine (an opioid receptor agonist). Shore crabs avoid shelter when they receive an electric shock and hermit crabs, which are soft-bodied and live in mollusc shells, will abandon a high-quality shell after a shock only to remain in a “safer” yet poorer-quality shell afterwards. Studies have also demonstrated a physiological stress response after potentially painful treatment in crabs. Few studies have performed a classic electrophysiological characterisation of nociceptors in crabs and we know relatively little about central nervous system responses. Therefore, it is imperative that we fill this knowledge gap.
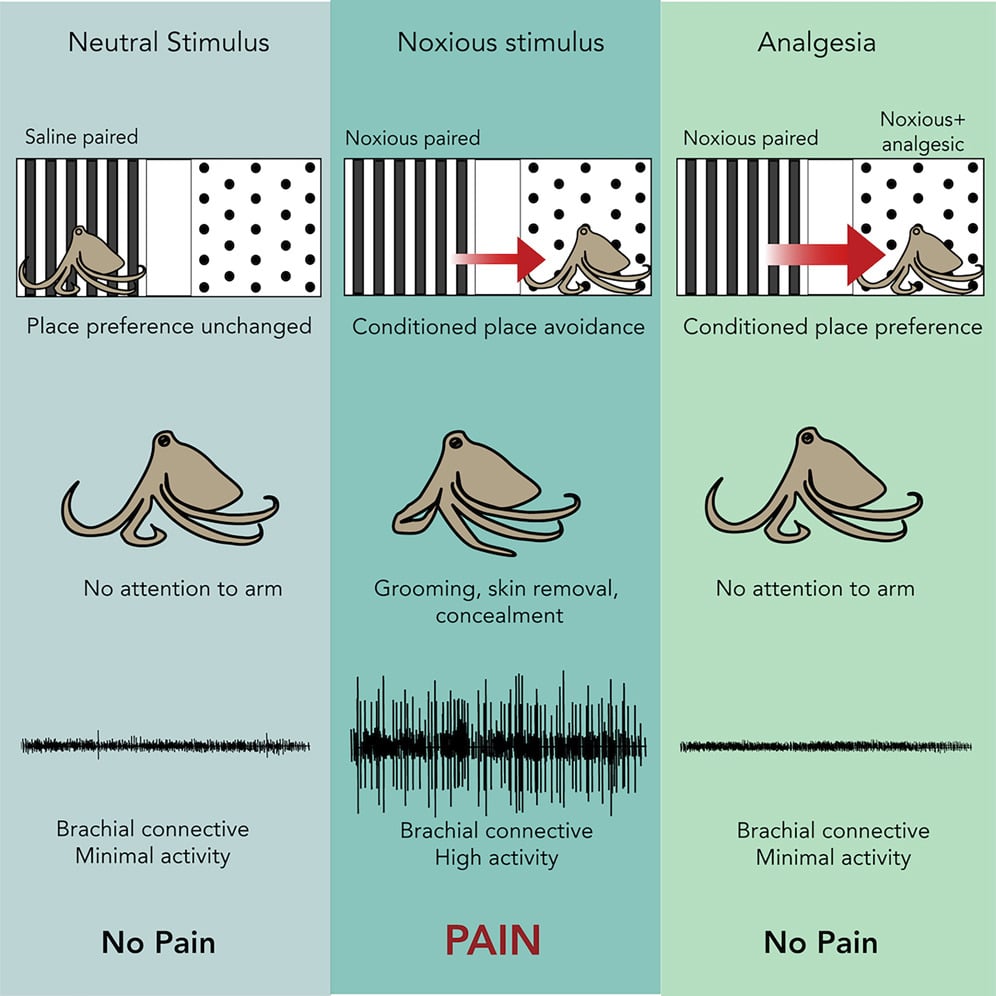
In contrast, nociceptors have been identified and recordings have been taken that demonstrate the evolutionary conservation of nociceptor properties in cephalopods. A very elegant study performed by Robyn Crook measures both behaviour changes and nociceptor activity in the octopus (Fig.4; Crook, 2021). Octopuses avoided areas where pain was experienced, demonstrating that pain is aversive, whereas they exhibited a preference for an area where they experienced pain relief. These animals also groomed the acetic acid injection site but this was prevented by the use of a pain relieving drug, the local anaesthetic lidocaine. Electrophysiological recordings demonstrated the presence of nociceptor activity when a pain stimulus was given and its absence after pain-relief administration. What this study tells us is that octopuses are motivated by pain and will avoid an area associated with it, so it must be a detrimental experience. Conversely, providing pain relief resulted in the octopus showing a clear preference for the area where pain was reduced by lidocaine administration, demonstrating that the relief of a negative internal affective state, pain, alters the animal’s preferences. Animals with no pain showed no preference for either area, demonstrating that pain was the motivational stimulus behind the animals’ behavioural decision-making (Fig.4).
Should we protect animals where there is evidence for pain?
Given the heightened awareness of animal welfare issues by the general public and the growing calls for inclusion of decapods and cephalopods into existing animal welfare legislation, decisions regarding other animal groups may rise to prominence. Governments and regulatory bodies will need scientific evidence to base their decisions on. This is a unique opportunity for physiologists to ensure their science makes a wider impact. There are many different opinions on whether non-human animals can experience pain. Irrespective of one’s own opinion, there are many advantages to ensuring good animal welfare for human benefit. In the context of experimental research, pain may present a confounding factor and so minimising pain after an invasive procedure ensures you are measuring the response to your experimental treatment rather than pain symptoms. Keeping animals in good welfare may lead to reduced individual variation, lower sample sizes and better reproducibility. When studying pain itself, animal models with more accessible nervous systems, which allow easier monitoring, can help us find novel ways of improving human pain treatment. Further, the work can provide new insights into the comparative biology and evolution of nociception and pain, not only adding to our understanding but informing decisions regarding the welfare of all animals.
References
Birch J et al. (2021). Review of the evidence of sentience in cephalopod molluscs and decapod crustaceans. https://www.lse.ac.uk/business/consulting/assets/documents/Sentience-in-Cephalopod-Molluscs-and-Decapod-Crustaceans-Final-Report-November-2021.pdf.
Crook RJ. (2021). Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience 24(3), 102229. https://doi.org/10.1016/j.isci.2021.102229.
Delvendahl N et al. (2022). Edible insects as food–insect welfare and ethical aspects from a consumer perspective. Insects 13(2), 121. https://doi.org/10.3390/insects1302012.
Elwood RW. (2019). Discrimination between nociceptive reflexes and more complex responses consistent with pain in crustaceans. Philosophical
Transactions of the Royal Society B 374(1785), 20190368. https://doi.org/10.1098/rstb.2019.0368.
Merskey H. (1979). Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain 6, 249-252.
McParland A et al. (2021). The brinker repressor system regulates injury-induced nociceptive sensitization in Drosophila melanogaster. Molecular
Pain 17, 17448069211037401. https://doi.org/10.1177/17448069211037401.
Raja SN et al. (2020). The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 161(9), 1976-1982. https://doi.org/10.1097/j.pain.0000000000001939.
Standing Committee on Agricultural Research. (2018). Strengthening fish welfare research through a gap analysis study. https://scar-europe.org/images/FISH/Documents/Report_CWG-AHW_CASA_FISH-welfare.pdf.
Sneddon LU. (2002). Anatomical and electrophysiological analysis of the trigeminal nerve in a teleost fish, Oncorhynchus mykiss. Neuroscience Letters 319(3), 167-171. https://doi.org/10.1016/S0304-3940(01)02584-8.
Sneddon LU. (2018). Comparative physiology of nociception and pain. Physiology 33, 63-73. https://doi.org/10.1152/physiol.00022.2017.
Sneddon LU. (2019). Evolution of nociception and pain: Evidence from fish models. Philosophical Transactions of the Royal Society B 374, 20190290. https://doi.org/10.1098/rstb.2019.0290.
Sotocina SG et al. (2011). The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Molecular Pain 7(55), 1744-8069. https://doi.org/10.1186/1744-8069-7-55.
