
Physiology News Magazine
The importance of being in the right spot
Glycogen is distributed differently in distinct fibre phenotypes, glycogen utilization is different at distinct localizations, and the role of glycogen in working skeletal muscles depends on its localization at a subcellular level.
Features
The importance of being in the right spot
Glycogen is distributed differently in distinct fibre phenotypes, glycogen utilization is different at distinct localizations, and the role of glycogen in working skeletal muscles depends on its localization at a subcellular level.
Features
Joachim Nielsen(1), Hans-Christer Holmberg(2), Henrik D. Schrøder(3), Bengt Saltin(4), Niels Ørtenblad(1)
1: Institute of Sports Science and Clinical Biomechanics and 3: Institute of Pathology, University of Southern Denmark, Odense, Denmark
2: Swedish Winter Sports Research Centre, Department of Health Sciences, Mid Sweden University, Sweden
4: Copenhagen Muscle Research Centre, University of Copenhagen, Copenhagen, Denmark
https://doi.org/10.36866/pn.87.23
In the subcellular architecture of skeletal muscle cells, glycogen is distributed heterogeneously next to contractile proteins and compartments responsible for energy delivery and ionic transmembrane flux. Recently, we have demonstrated that glycogen is distributed differently in distinct fibre phenotypes, glycogen utilization is different at distinct localizations, and the role of glycogen in working skeletal muscles depends on its localization at a subcellular level. Thus, considerations of the subcellular localization have implications for the understanding of glycogen metabolism in skeletal muscle cells.
Glycolysis occurs in virtually all living organisms, both in the absence and presence of oxygen. The wide occurrence of glycolysis suggests that it is one of the most ancient metabolic pathways, providing a primary mechanism by which energy from glucose can be converted to ATP and drive fundamental cellular processes. Glycogen is a branched polymer of glucose and the substance in which cells store and mobilise glucose, providing fuel for the glycolysis. Glycogen hence stores and provides the fuel (i.e. glucose) for glycolysis. In connection with most forms of work performed by skeletal muscle, glycogen is a major source of energy and, indeed, depletion of glycogen is strongly associated with exhaustion (Bergstöm et al. 1967). Despite the fundamental acceptance of this vital role of glycogen, the underlying mechanism(s) still remain unknown.
In almost all previous investigations glycogen levels in muscle have been determined using muscle homogenates, which provides an overall or average value. However, it is becoming increasingly clear that glycogen is not evenly distributed in muscle cells. For instance, transmission electron microscopy (TEM) reveals that glycogen exists in the form of discrete particles that are distributed heterogeneously within the cell (Wanson & Drochmans, 1968).
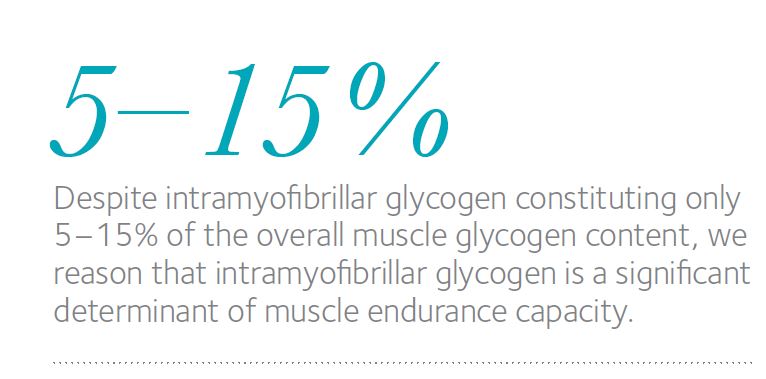
The structural organization of skeletal muscle cells consists of contractile filaments arranged in thousands of parallel longitudinally oriented myofibrils that occupy 70–80% of the cell volume. Interspersed between these myofibrils are networks of mitochondria and triads of sarcoplasmic reticulum, as well as invaginations of the surface membrane, the so-called T-tubuli, which together create an intermyofibrillar space. In this cellular architecture, glycogen is distributed both within the myofibrils (intramyofibrillar (Intra) glycogen) and between the myofibrils in close proximity to mitochondria and sarcoplasmic reticulum (intermyofibrillar (IMF) glycogen). In addition, glycogen is also found just beneath the surface membrane primarily near the mitochondria and nuclei (subsarcolemmal (SS) glycogen). Until now, very little information was available regarding glycogen localization in skeletal muscle: an observational study suggested that the utilization of glycogen during exercise is localization dependent (Fridén et al. 1985) and one quantitative study showed a preferential increase in Intra glycogen during post-exercise recovery (Marchand et al. 2007).
Recent studies in our laboratory were performed to investigate by quantitative unbiased methods the localization of glycogen before and after exercise in muscle fibres of different phenotype (Nielsen et al. 2011), and the role that glycogen localization plays in muscle function in vitro (Nielsen et al. 2009; Ørtenblad et al. 2011).
Both prior to and following a high-intensity cross-country race lasting approximately 1 h, biopsies were taken from the highly trained arm muscle (triceps brachii) of 10 elite Norw .egian cross-country skiers (22 ± 1 years,VO2max = 68 ± 5 ml kg-1 min-1 (means ± SD)). TEM revealed that before this race 75–85% of the glycogen was located in the intermyofibrillar space, whereas only 5–15% was present in each of the intramyofibrillar space and subsarcolemmal space (Nielsen et al. 2011). The oxidative type I fibres contained 80% more Intra glycogen than the glycolytic type II fibres (Fig. 1). Thus, in elite athletes training in an endurance discipline, the distribution of endogenous glycogen in skeletal muscle fibres of two distinctive phenotypes differs.
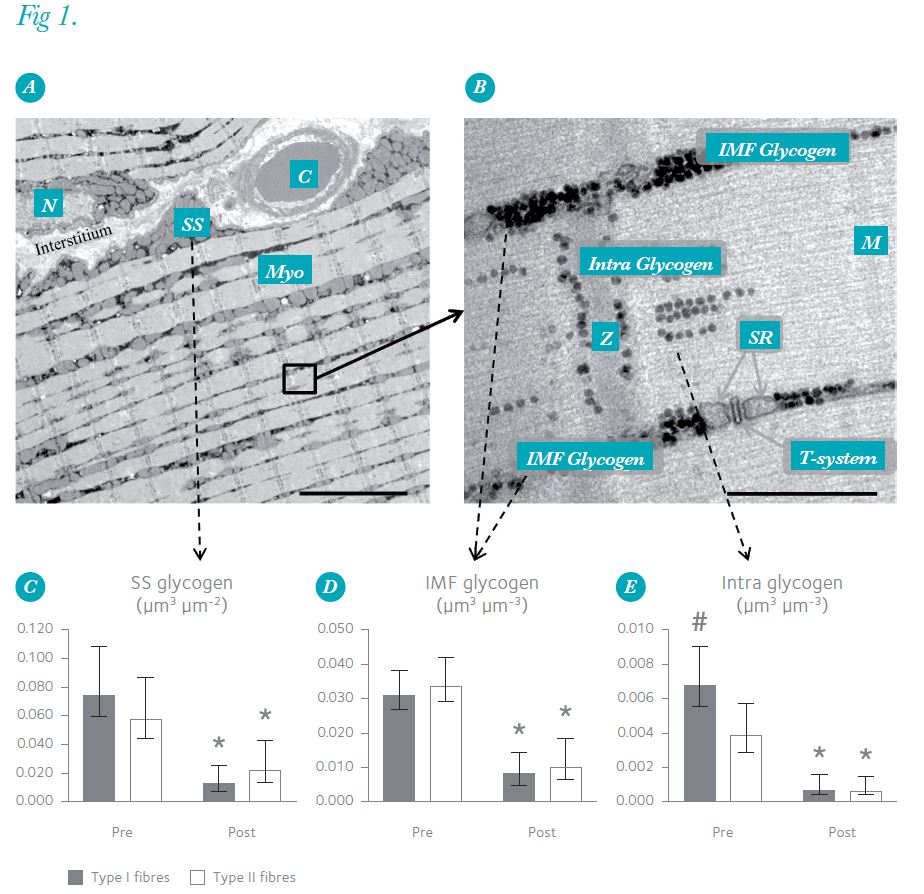
After the race, the overall muscle content of glycogen, determined biochemically, was 70% lower than before this exertion. However, the levels in different subcellular fractions were reduced to varying degrees (Fig. 1): the minor depot of Intra glycogen fell by 85%, while the decreases in IMF and SS glycogen were only 70%. Consequently, the relative contribution of Intra glycogen to the overall muscle glycogen content decreased from 11% before the race to 6% after the race in the oxidative type I fibres, and from 7% to 4% in the glycolytic type II fibres, demonstrating that Intra glycogen was preferentially used during the race in both type I and type II fibres (Nielsen et al. 2011).

Despite Intra glycogen constituting only 5–15% of the overall muscle glycogen content, we reason that Intra glycogen is a significant determinant of muscle endurance capacity. This is based on the two findings – i.e. a preferential depletion of Intra glycogen during exercise and more Intra glycogen in the relatively fatigue-resistant, oxidative fibres than in the glycolytic fibres. Further support for this conclusion is provided by our two recently published in vitro observations that the level of Intra glycogen influences the capacity of rat muscle fibres to resist fatigue (Nielsen et al. 2009) and that a low level of Intra glycogen is associated with impaired release of Ca2+ by the sarcoplasmic reticulum, which is considered to contribute to muscle fatigue (Ørtenblad et al. 2011).
Together, our observations indicate that the relatively minor depot of glycogen located in the intramyofibrillar space plays an important role in muscle function. Although the mechanistic explanation for this significance of glycogen compartmentalization remains to be elucidated, these findings are intriguing with regards to possible co-localization of glycogen and compartmentalized energy transfer through glycolysis, as well as the function of glycogen-associated proteins in cellular processes. This may be of crucial importance in cells with a high and fluctuating metabolism, including excitable tissues such as muscles, the heart and nerves.
References
Bergström J, Hermansen L, Hultman E & Saltin B (1967). Diet, muscle glycogen and physical performance. Acta Physiol Scand 71, 140–150.
Fridén J, Seger J & Ekblom B (1985). Implementation of periodic acid-thiosemicarbazide-silver proteinate staining for ultrastructural assessment of muscle glycogen utilization during exercise. Cell Tissue Res 242, 229–232.
Marchand I, Tarnopolsky M, Adamo KB, Bourgeois JM, Chorneyko K & Graham TE (2007).Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol 580, 617–628.
Nielsen J, Holmberg H-C, Schrøder HD, Saltin B & Ørtenblad N (2011). Human skeletal muscle glycogen utilization in exhaustive exercise: Role of subcellular localization and fibre type. J Physiol 589, 2871–2885. http://jp.physoc.org/content/589/11/2871.long
Nielsen J, Schrøder HD, Rix CG & Ørtenblad N (2009). Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587, 3679–3690.
Ørtenblad N, Nielsen J, Saltin B & Holmberg H-C (2011). Role of glycogen availability on SR Ca2+ kinetics in human skeletal muscle. J Physiol 589, 711–725.
Wanson JC & Drochmans P (1968). Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen beta-particles isolated by the precipitation-centrifugation method. J Cell Biol 38, 130–150.
