
Physiology News Magazine
The neuroscience of balance
From athletes to the elderly
Features
The neuroscience of balance
From athletes to the elderly
Features

https://doi.org/10.36866/pn.126.28
Dr Kathleen E Cullen
Johns Hopkins University, US
Aristotle described five senses, namely sound, sight, touch, smell, and taste, that provide us with a conscious awareness of the world around us. But he missed one of our most important senses: the vestibular (inner ear) system, which makes critical contributions to our sense of balance. In this article, I will focus on how single neurons, organised into dedicated circuits, support our ability to stay on our feet.
The vestibular system – a 6th sense
The vestibular system detects head motion to generate essential reflexes required for stable gaze and balance, and to provide us with our sense of motion relative to our world (self-motion) and spatial orientation. This essential sensory system is located in the inner ear just next to the cochlea, within the petrous part of the temporal bone (Fig.1).
In mammals, the vestibular system comprises five sensory organs on each side of the head, namely (i) the three fluid-filled semicircular canals, which sense rotational head acceleration in three axes and (ii) the two otolith organs (the saccule and utricle), which sense linear head acceleration (i.e. gravity and translational movements).
Accordingly, the vestibular system detects and encodes the angular and linear components of motion separately, with the semicircular canals and otoliths functioning as miniature gyroscopes and linear accelerometers that provide online feedback about our current head motion during our daily activities.

Rapid multimodal integration ensures stable gaze and posture
As we explore our environment, the brain takes advantage of information from multiple sensory systems – vestibular, proprioceptive, and visual – to keep track of our movement and spatial orientation. In turn, the integration of this multimodal information underlies our ability to rapidly generate robust behaviours to remain stable and upright.
In this context, the vestibular system is incredibly fast. This is because the vestibular system is unique among sensory systems in that the same neurons that receive peripheral afferent nerve input, also send direct projections to motoneurons and motor centres. Likewise, the proprioceptive system, which senses the relative position of arms, legs, etc. in relation to each other, is also remarkedly fast. Indeed, when we experience unexpected self-motion, the vestibular and proprioceptive systems work together to generate compensatory postural responses within ~10 ms (reviewed in Cullen, 2019).
By comparison, the visual system’s contribution to behaviour is an order of magnitude slower (~100 ms) due to the inherent delays of the pathways that transform visual information into movement. Because patients with complete vestibular loss must rely more strongly on vision during everyday activities such as walking, they demonstrate impaired gaze and postural stability.
Accordingly, during everyday life, the vestibular and proprioceptive systems effectively work together to provide us with our essential “6th sense”. Indeed, when the vestibular system is functioning normally, we are unaware of a distinct sensation because vestibular information is integrated with proprioceptive and other sensory inputs to encode sense of motion.

via Wikimedia Commons)
Did I really want to do that?
The brain combines vestibular and proprioceptive information to maintain balance. But the brain also needs to constantly keep track of the vital question: Did I really want to do that?
Consider the fact that both unexpected motion and intended motion relative to the world will result in activation of the vestibular system (Fig.2). If the goal is to maintain posture, then the brain will use this sensory information to generate the postural adjustments required to stabilise ourselves relative to our environment via vestibulo-spinal pathways. For instance, the unexpected motion experienced when slipping on ice will activate the vestibulo-spinal pathways to generate rapid compensatory postural responses. Alternatively, the requirements are different when we voluntarily move through the world. While we will also experience vestibular sensory input, the resulting activation of the vestibular system is a consequence of our own behaviour and thus represents motion that was intended. Thus, in this case, it would actually be counterproductive for the brain to generate postural stabilising responses, since our goal is to move through rather than stabilise ourselves relative to the world. Accordingly, the efficacy of vestibulo-spinal pathways is modulated in a manner that depends on whether the experienced self-motion is the result of unintended or intended behaviour.
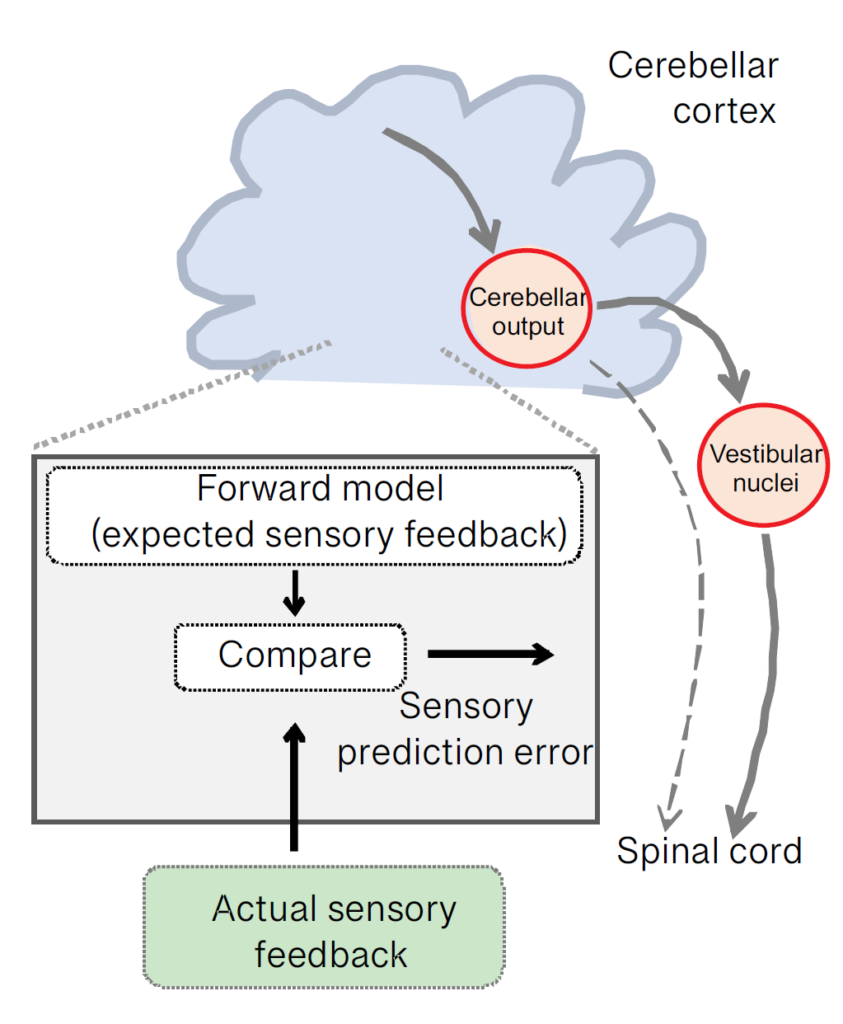
Indeed, we now know that during intended behaviours the brain computes an “internal model” (Fig.3) of the expected sensory consequences of our self-motion (Brooks et al., 2015). The computation is performed in the cerebellum, an area deep in the brain that plays an important role in the coordination of movement and balance. During our everyday activities, the cerebellum plays an essential role in the real-time regulation of movement required for coordinated behaviour and maintenance of posture. For instance, the act of suddenly slipping on ice would effectively produce a mismatch between the brain’s expectation of sensory input and the actual sensory input that is experienced. In response to such a mismatch, vestibulo-spinal pathways quickly send a robust signal to the spinal cord to maintain balance, which (hopefully) allows us to stay on our feet (Brooks and Cullen, 2013).
Accordingly, the cerebellum’s internal model allows us to effectively distinguish sensory input caused by our own actions versus unexpected self-motion. Athletes can compute this mismatch for very complex motions – consider a gymnast doing a back flip on a balance beam. In contrast, patients with severely impaired cerebellar function – due to either hereditary or acquired conditions – cannot do this, even for a simple movement like placing a foot on a step.
In addition to being important for postural control, the ability to distinguish between self-generated and externally applied stimuli is also vital to ensure perceptual stability. Notably, in 1867, Helmholtz made the salient observation that tapping on the canthus of the eye results in an illusionary shift of the visual world. However, we never see the world “shift” when we make rapid active eye movements (i.e. saccades) to look from one side of the room to the other. Indeed, the visual world remains stable because the brain can predict and suppress the visual stimulation produced by these active eye movements. Similarly, self-generated tactile stimulation in human subjects does not result in the same tickling sensation that arises when stimulation is externally produced (Witney et al., 1999). In the context of balance, single-unit recordings from the thalamocortical vestibular pathway have further shown that neurons selectively encode unexpected motion, thereby providinga neural correlate for ensuring perceptual stability during active versus externally generated motion (Dale and Cullen, 2017).
Thus, overall, the computation of a mismatch between expected and actual vestibular input is necessary for robust postural control and our subjective awareness of self-motion as we explore the world. Elite athletes demonstrate superior performance in their abilities to anticipate the sensory consequences of motor commands when learning new complex actions (reviewed in Yarrow et al., 2009). In contrast, as discussed below this ability declines as we age.
The role of motor signals in sensory processing
Recent experiments have provided circuit-level insight into the question of where and how the brain compares expected and actual sensory feedback. As we move through our world, cerebellar output neurons dynamically track the difference between the predicted versus actual sensory inflow that is experienced (Fig.3). As a result, these neurons effectively compute a representation of the unexpected motion that is experienced, which in turn is then used within milliseconds to adjust our balance. This signal is then relayed to vestibulo-spinal neurons that connect the cerebellum to the spinal cord to drive rapid compensatory postural responses to “unexpected” motion (Cullen, 2019). By repeatedly practising a complex action the brain can build sophisticated internal models of the expected feedback—as demanded by sports such as gymnastics and artistic pursuits such as dance.
Additionally, the brain learns to update its cerebellar internal model of what to expect, as our muscles and bodies change. Recordings from single cerebellar output neurons have demonstrated this updating in real time. Such updating achieves the flexibility required to continuously calibrate relationships between motor signals and the resultant sensory feedback across our life span.
Ageing and balance
Over our life span, we lose an increasing percentage of the vestibular receptor cells that we had when we were younger (Fig.4). Thus, much like how we lose hearing with age, we also lose our vestibular sense. As a result, we have less reliable vestibular sensory information to estimate how we are moving relative to world to maintain balance. Further, as we age, we typically become more sedentary, and as we move less our brains often learn to rely more on vision. However, this is not ideal since, as noted above, vision is a very slow sensory input compared to vestibular and proprioceptive systems. In patients with vestibular loss, rehabilitation programmes focus on retraining the brain’s strategy for integrating sensory information so that it learns to again use faster sensory inputs such as the vestibular system and proprioception to better maintain balance. Similar training as well as exercises such as Tai chi and Pilates have also proven to be beneficial for improving balance in the elderly and are thought to upweight the brain’s reliance on self-motion information from the vestibular and proprioceptive systems (Sun et al., 2021).

Cognitive aspects of vestibular disorders
The ability to form a picture of where we are heading relative to where we currently are requires knowledge of our current location and the direction of our self-motion. There is evidence to show that the hippocampus plays a vital role in such spatial navigation, and that the vestibular system provides a key input (Brandt et al., 1994). Interestingly, there is also increasing evidence that cognitive decline in patients with Alzheimer’s disease is linked to peripheral vestibular loss observed during ageing. For example, patients with cognitive impairment have poorer vestibular function (most notably, impaired otolith responses) relative to age-matched controls (Harun et al., 2016). Understanding the links between peripheral vestibular loss and cognitive impairment in disease, as well as in normal ageing will be an essential direction for basic and clinical future research.
Conclusion
The brain’s ability to distinguish externally applied from self-generated sensory inputs underlies our ability to achieve both perceptual stability and accurate motor control during everyday activities. Recent studies have provided circuit-level insight into the neural computations performed in vestibular pathways to ensure these vital functions. Nevertheless, several open questions remain regarding the computations that the brain performs on vestibular information to ensure stable perception and accurate motor control. For example, further studies are needed to establish exactly how the cerebellum builds a neural representation of the expected sensory consequences of our voluntary self-motion. Additionally, further research is required to elucidate how functional changes in cerebellar versus higher-level cortical processing contribute our ability to maintain balance across our life span.
Ultimately, an improved understanding of these vestibular neural mechanisms can be leveraged to advance the development of more targeted training and rehabilitation strategies to counter deficits due to neurological disorders and/or ageing. Moreover, such fundamental knowledge can be leveraged to determine the extent to which the performance of complex movements can be optimised through extensive practice versus to what extent innate inter-individual differences simply make elite athletes different from the rest of us.
References
Brandt T et al. (1994). Vestibular cortex lesions affect the perception of verticality. Annuals of Neurology 35, 403–12. https://doi.org/10.1002/ana.410350406.
Brooks JX, Cullen KE (2013). The primate cerebellum selectively encodes unexpected self-motion. Current Biology 11, 947-55. https://doi.org/10.1016/j.cub.2013.04.029.
Brooks JX et al. (2015). Learning to expect the unexpected: Rapid updating in primate cerebellum during voluntary self-motion. Nature Neuroscience 18, 1310–7. https://doi.org/10.1038/nn.4077.
Cullen KE (2019). Vestibular processing during natural self-motion: Implications for perception and action. Nature Reviews Neuroscience 20, 346-363. https://doi.org/10.1038/s41583-019-0153-1.
Dale A, Cullen KE (2017). The ventral posterior lateral thalamus preferentially encodes externally applied versus active movement: Implications for self-motion perception. Cerebral Cortex 29, 1–14. https://doi.org/10.1093/cercor/bhx325.
Harun A et al. (2016). Vestibular impairment in dementia. Otology & Neurotology 37,1137–42. http://dx.doi.org/10.1097/MAO.0000000000001157.
Rauch SD et al. (2001). Decreasing hair cell counts in aging humans. Annals of the New York Academy of Sciences 942, 220-7. https://doi.org/10.1111/j.1749-6632.2001.tb03748.x.
Sun M et al. (2021). The effect of exercise intervention on reducing the fall risk in older adults: A meta-analysis of randomized controlled trials. International Journal of Environmental Research and Public Health 18(23),12562 https://doi.org/10.3390/ijerph182312562.
Witney AG et al. (1999). Predictive motor learning of temporal delays. Journal of Neurophysiology 82(5):2039-48. https://doi.org/10.1152/jn.1999.82.5.2039.
Yarrow K et al. (2009). Inside the brain of an elite athlete: The neural processes that support high achievement in sports. Nature Reviews Neuroscience 10, 585–596. https://doi.org/10.1038/nrn2672.
