
Physiology News Magazine
The orderly recruitment of postganglionic sympathetic neurons
Investigations into discharge patterns of the multi-unit postganglionic sympathetic neural population in humans have been hampered by the poor signal-to-noise aspect of this signal. New signal processing and denoising approaches have now been used to expose such action potential patterns. Such approaches have revealed that variations exist in the conduction velocity of action potentials observed in this neurogram and that a subpopulation of fast-conducting neurons exists for probable recruitment during high stress scenarios.
Features
The orderly recruitment of postganglionic sympathetic neurons
Investigations into discharge patterns of the multi-unit postganglionic sympathetic neural population in humans have been hampered by the poor signal-to-noise aspect of this signal. New signal processing and denoising approaches have now been used to expose such action potential patterns. Such approaches have revealed that variations exist in the conduction velocity of action potentials observed in this neurogram and that a subpopulation of fast-conducting neurons exists for probable recruitment during high stress scenarios.
Features
J. K. Shoemaker(1,2), A. Salmanpour(1) and C. D. Steinback(1)
1: Neurovascular Research Laboratory, School of Kinesiology
2: Department of Physiology and Pharmacology, The University of Western Ontario, London, Ontario, Canada N6A 3K7
https://doi.org/10.36866/pn.84.14
Order is the shape upon which beauty depends
Pearl S. Buck (1892–1973)



Blood pressure and blood flow are regulated, to a large extent, by the sympathetic nervous system through its influence on the function of the heart and blood vessels. This influence is exerted through varying levels of sympathetic nerve activity (SNA) and the consequential emission of a variety of chemicals or neurotransmittors. The level of SNA may increase rapidly in response to stressors (the ‘fight-or-flight’ response), such as changes in posture, emotional arousal, fatiguing exercise and others. Yet, the corresponding cardiovascular responses may vary with the type of reflex. Also, current evidence indicates that the levels of SNA at baseline and during a reflex response are affected by one’s sex, age and disease state. Physiologically, there is considerable uncertainty surrounding the manner in which SNA is emitted and how variations in its discharge patterns are regulated, particularly in conscious humans where direct access to neural recordings from the sympathetic nervous system is limited. Nonetheless, variations in SNA discharge are likely to reflect mechanistic determinants of sympathetic recruitment as well as provide an important input to cardiovascular control.
Historically, a major breakthrough in measuring and quantifying SNA discharge patterns in humans came in the late 1960s when adaptations to microneurographic techniques enabled direct recordings from postganglionic sympathetic neurons (Hagbarth & Vallbo, 1968). Using tungsten electrodes penetrating groups of sympathetic neurons in peripheral nerves, such as the peroneal (fibular) nerve, it was learned that SNA directed to blood vessels within skeletal muscle (MSNA) exhibits bursty behaviour, with groups of axons discharging more-or-less simultaneously in a manner that is entrained to the cardiac cycle. There is variation in the rate and size of these bursts, suggesting a corresponding modulation of neural recruitment. However, due to the relatively poor signal-to-noise aspects of the SNA signal from human peripheral nerves, analysis of this multi-fibre recording has, to a large extent, been constrained to the integrated neurogram. Through band-pass filtering, rectification and integration, SNA data are typically smoothed to reduce background noise and provide a quantitative measure of sympathetic outflow in terms of the number and size of integrated bursts. However, this approach eliminates all information from individual neurons and the action potentials (APs; often called ‘spikes’) that make up the total signal. This places a constraint on the ability to study sympathetic neural discharge patterns and recruitment strategies, as well as on the ability to understand the mechanisms linking SNA and end-organ control in humans.
For example, a provocative observation in the integrated neurogram from human MSNA recordings is that the conduction of sympathetic traffic, based on the delay of the burst from a representative R-wave of the electrocardiogram (Wallin et al. 1994), is inversely related to burst size. The shorter reflex latency of larger bursts is hypothesized to be due to (a) variations in synaptic delays between the brainstem and peripheral nerve that produce more discharges of the same neurons per burst, and/or (b) more than one population of efferent sympathetic neurons with progressive recruitment thresholds and conduction velocities (Wallin et al. 1994). Although articulated in 1994, these issues have remained untested in human or smaller animal models due to methodological limitations for examining SNA at the AP level.
Since 1994, two experimental approaches have been advanced to address SNA discharge patterns and their control in humans. First, the use of higher impedance electrodes enabled the study of single neurons and their behaviour over time. These studies demonstrated that postganglionic sympathetic axons discharge in a probabilistic manner, typically firing only once in a burst of sympathetic activity (Macefield et al. 1994); however, multiple within-burst firings of the same neuron may occur with increasing stress (Macefield & Wallin, 1999). From these studies on single-fibre recordings, Macefield proposed that increases in firing probability of active neurons may be the primary mechanism by which integrated burst intensity (size) is augmented. It is also possible that a population of latent neurons is available for recruitment during stress. Although both of these potential mechanisms have been observed in other neural systems, such as the skeletal-motor system (Henneman et al. 1965), such ideas regarding sympathetic neural recruitment required analysis of the multi-unit firing patterns rather than one neuron at a time.
More recently, the advancement of wavelet-based denoising approaches has opened new opportunities to study the patterns of AP discharge within each burst of sympathetic activity. This approach has led to reports of variations in postganglionic sympathetic AP rate, both at rest and during reflex activation (Diedrich et al. 2003). However, the ability to study the presence (or absence) of differing populations of postganglionic sympathetic neurons with varying recruitment thresholds or patterns requires additional understanding of the timing and size of APs along with subsequent analysis of properties related to neuronal size, such as its conduction velocity. Recently, our laboratory published new information regarding such discharge patterns using an improved AP detection and classification approach (Salmanpour et al. 2010) (see Fig. 1).
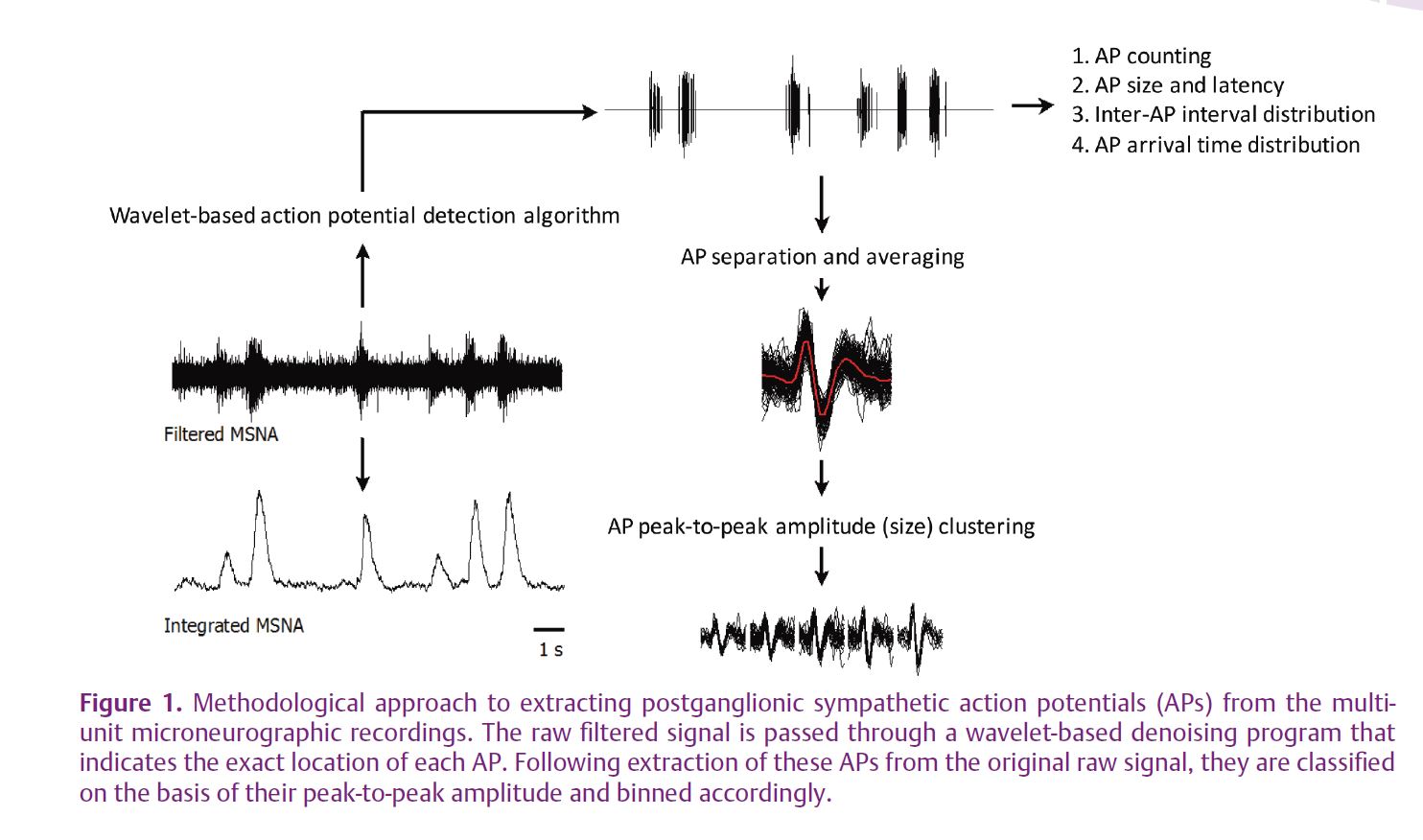
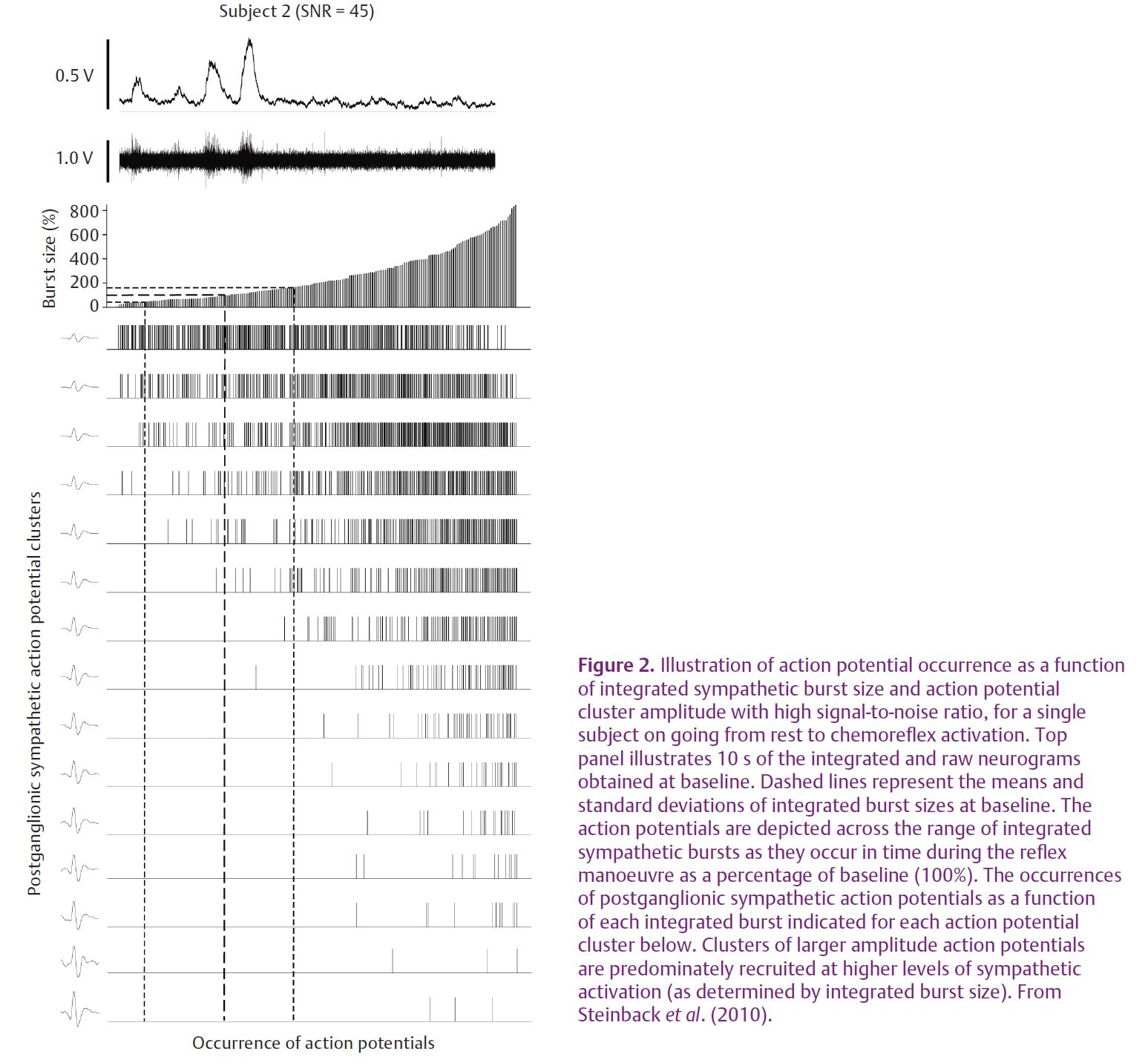
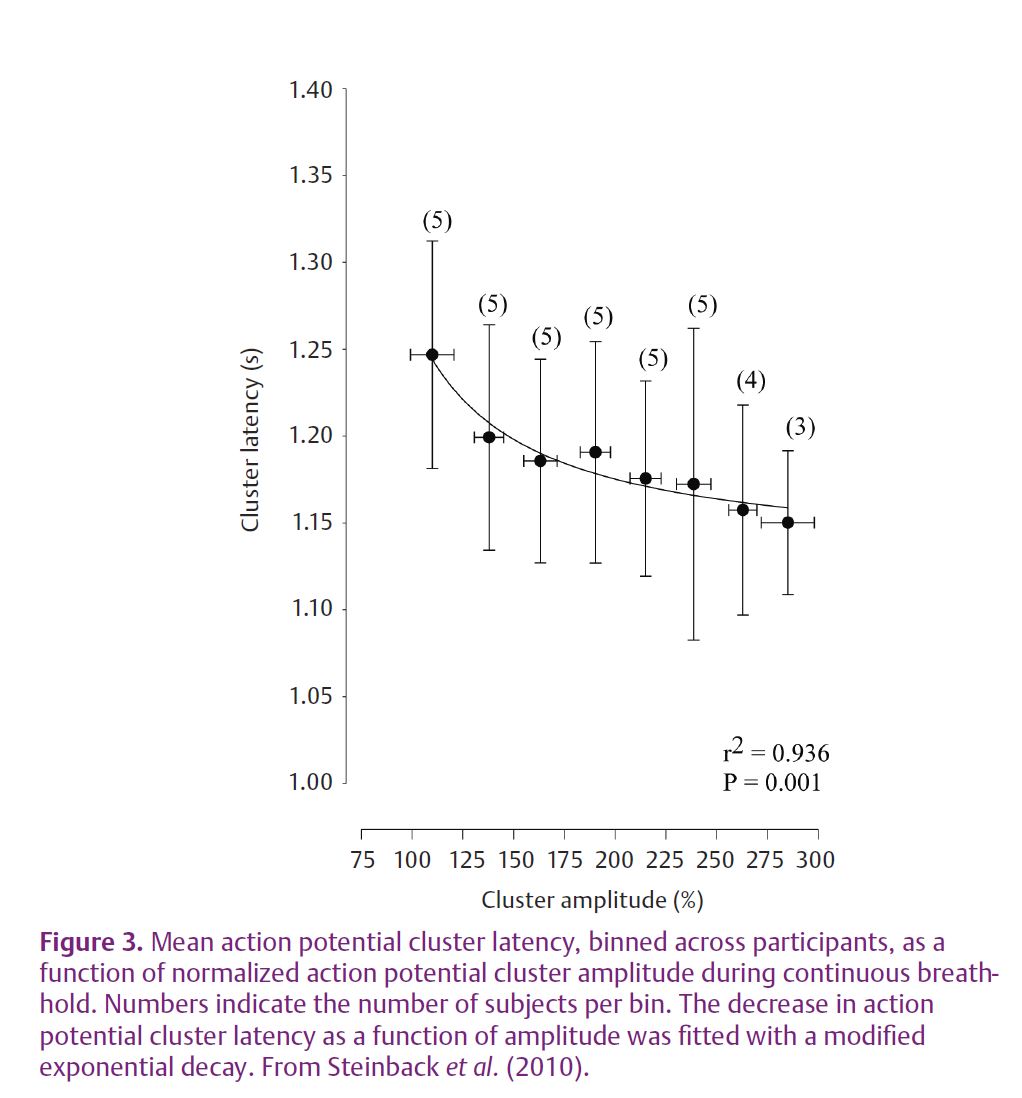
With this approach we have studied the question of AP content within bursts of SNA of various sizes and the recruitment of new neurons during severe physiological stress (Steinback et al. 2010). Very large increases in muscle SNA are observed during severe chemoreflex stress due to increases in both burst frequency and size. Such a reflex response was considered ideal for the study of AP patterns across a range of reflex stress. Severe chemoreflex stress was produced by a prolonged breath-hold performed by trained free divers. The illustration of the AP discharge pattern as a function of integrated burst size and location are presented in Fig. 2 for a single individual. As a group, these individuals held their breath for 178 ± 37 s resulting in a haemoglobin saturation of 80 ± 11% and arterial CO2 partial pressures of 51 ± 3 Torr. The average amplitude of each MSNA burst increased from 0.24 ± 0.04 V at rest to 1.34 ± 0.38 V at maximum breath-hold and the average number of APs per burst of muscle SNA increased from 14 ± 7 at rest to 40 ± 12 at maximum breath-hold. Moreover, we demonstrated that the number of active AP clusters (representing discreet populations of neurons of a particular size) per burst of muscle SNA increased from 4 ± 2 clusters per burst at rest to 13 ± 3 clusters per burst at maximum stress. Importantly, larger APs became increasingly evident in the muscle SNA neurogram as the chemoreflex stress increased and as integrated burst size increased. In addition, the latency of these APs, relative to their corresponding R-wave, was inversely related to the size of the AP cluster. Thus, the larger APs demonstrated a faster conducting velocity. When considered over the group and breath-hold period, a distinct decaying exponential relationship between AP amplitude and reflex latency became evident (Fig. 3).
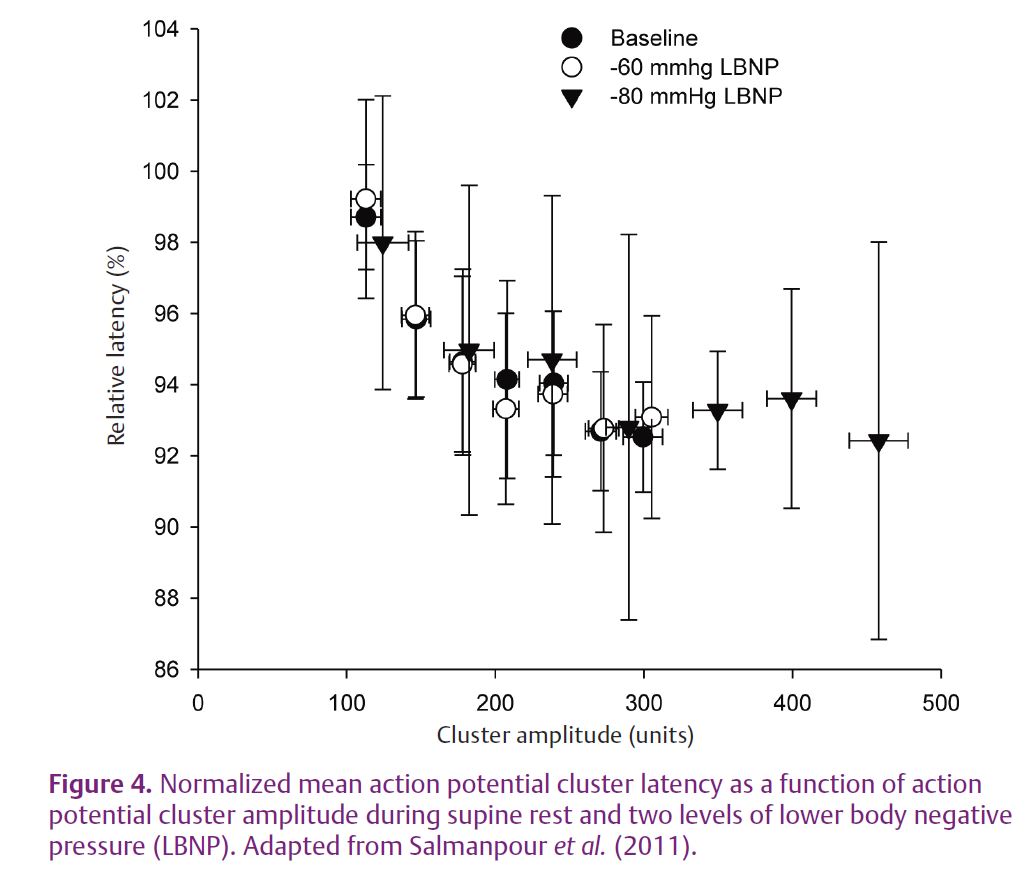
The above data were obtained during the progression of chemoreflex stress on the expectation that large APs would be more likely to appear in severe reflex sympathoexcitatory scenarios. However, there is a large variation in integrated MSNA burst size even under conditions of supine rest when physiological stress is considered to be minimal. Also, other reflexes, such as baroreflex activation during postural challenges (in response to a rapid drop in blood pressure), increase the rate of integrated bursts of MSNA but have less impact on the size of these bursts. Therefore, we have also taken a closer look at AP characteristics both at rest and during high levels of lower body negative pressure to examine the influence of MSNA burst size alone and with baroreflex unloading on such discharge patterns (Salmanpour et al. 2011) (Fig. 4). The results indicate that the inverse hyperbolic relationship between AP cluster size and its conduction latency, observed previously during chemoreflex stimuli, is present both at rest and during –60 and –80 mmHg of lower body negative pressure. However, what is significant is the introduction of larger APs at the high levels of negative pressure, at least in some of the individuals.
Overall, when these new data are coupled with reports from single-neuron recordings, analysis of multi-unit AP patterns and morphology exposes a pattern of regulatory order in postganglionic neuronal recruitment. Specifically, one mechanism of increasing MSNA is to increase the firing rate of already-recruited axons as indicated by increasing numbers of similar-sized APs per burst of SNA. However, there also appears to be a latent population of neurons available for recruitment. Some of these latent neurons are active at baseline but are likely to be expressed in the larger bursts of MSNA. Therefore, the expression of larger bursts of MSNA at any time occurs because of the increased number of APs and the appearance of new and larger AP clusters. Moreover, the likelihood of these larger APs and additional clusters of larger APs being present in the neurogram appears to increase during severe reflex stress. Importantly, the larger AP clusters are characterized by a shorter reflex latency which supports the premise that they represent larger axons with faster conduction velocities. Thus, this work suggests that larger and faster-conducting postganglionic sympathetic neurons (a) are largely latent at baseline, (b) are available for recruitment, (c) have a higher probability of recruitment during high physiological stress, and (d) are present in larger rather than smaller bursts of MSNA. As this pattern is analogous to previously defined patterns of motor neuron activation, it appears that this ordered recruitment pattern may be a fundamental feature of excitable neural systems.
References
Diedrich A, Charoensuk W, Brychta RJ, Ertl AC & Shiavi R (2003). Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng 50, 41–50.
Hagbarth KE & Vallbo AB (1968). Pulse and respiratory grouping of sypmathetic impulses in human sympathetic nerves. Acta Physiol Scand 74, 96–108.
Henneman E, Somjen G & Carpenter DO (1965). Functional significance of cell size in spinal motoneurons. J Neurophysiol 28, 560–580.
Macefield VG & Wallin BG (1999). Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516, 293–301.
Macefield VG, Wallin BG & Vallbo AB (1994). The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481, 799–809.
Salmanpour A, Brown LJ & Shoemaker JK (2010). Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. J Neurosci Methods 193, 343–355.
Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R & Shoemaker JK (2011). Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. J Neurophysiol (in press).
Steinback CD, Salmanpour A, Breskovic T, Dujic Z & Shoemaker JK (2010). Sympathetic neural activation: an ordered affair. J Physiol 588, 4825–4836.http://jp.physoc.org/content/588/23/4825.long
Wallin BG, Burke D & Gandevia SC (1994). Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 474, 331–338.
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada.
