Physiology News Magazine
The peri-conceptional origins of the life-long physiological consequences of being a twin
Studies in twins, both human and ovine, suggest that the fetal developmental trajectory in twins is different from that of singletons and may be reflected by altered physiology in post-natal life. The early pregnancy environment may be critical in determining both pregnancy outcome and the risk for adverse long-term health outcomes
Features
The peri-conceptional origins of the life-long physiological consequences of being a twin
Studies in twins, both human and ovine, suggest that the fetal developmental trajectory in twins is different from that of singletons and may be reflected by altered physiology in post-natal life. The early pregnancy environment may be critical in determining both pregnancy outcome and the risk for adverse long-term health outcomes
Features
Frank H Bloomfield
Liggins Institute, University of Auckland, Private Bag 92019, Auckland, New Zealand
https://doi.org/10.36866/pn.75.31

Pregnancy outcome in twins
The incidence of twin (and higher order multifetal) pregnancies is increasing worldwide, largely due to increased use of assisted reproductive techniques and advancing maternal age. Twins are born both earlier and smaller than singletons with a mean gestation length of 37 weeks compared with 40 weeks in singletons. The reduced size at birth in twins is not only due to their shorter gestational length; rather, twins have a different birth-size distribution (affecting weight, length and head circumference) from singletons and this persists into childhood.
In essence, therefore, twins could be considered to have constrained fetal growth in that they fail to grow to their genetic potential. This is also the definition of intrauterine growth restriction (IUGR). Do a mean gestational length decrement of 3 weeks, and a shift to the left in distribution of birth size in twins matter?
The consequences of being a twin for short- and long-term health outcomes
Newborn twins have a higher incidence of most common neonatal problems, with mortality and disability rates increased 5- to 10-fold compared with those of singletons. Much of the increased risk can be attributed to preterm birth or IUGR, with even late preterm birth (birth at 34–36 weeks gestation) now recognised as carrying significantly increased risks for a variety of adverse outcomes, including not only physiological parameters such as blood pressure and glucose tolerance, but also developmental progress and school achievement (Morse et al. 2009).
The associations between reduced size at birth and a large range of adverse health outcomes in adulthood are now well established in singletons. However, the literature on these associations in twins is much less clear. This may be due in part to classical methodological approaches in twin studies, which entail comparisons of outcomes between monozygotic and dizygotic twins to separate the effects of genes versus environment. However, when the effects of the intrauterine environment on outcome are being studied, this separation is not so distinct. The intrauterine environment is affected by two components: maternal factors (e.g. nutritional status, body habitus, ingestion of toxins), which will be common to both fetuses; and factors intrinsic to each fetus. Some of these fetal factors will be genetic, but others will not. For example, the proportion of the placental nutrient supply that each member of a monozygotic twin pair claims is not related to genotype. More recent studies have addressed this issue by including coefficients for both within twin-pair and between twin-pair factors in the statistical analyses, and these studies have tended to find the same associations between birth weight and risk of adult disease in twins.
The question of the effect of a twin pregnancy on outcome is not one that most animal experiments can address. The majority of the animal studies investigating the associations between intrauterine environment and long-term outcomes have been performed in polytocous species
(those that give birth to several fetuses at once). Furthermore, these species often give birth to young that undergo critical aspects of organ development (such as of the pancreas) postnatally, rather than before birth as in the human. Clearly, if fetuses from multifetal pregnancies are fundamentally different from singleton fetuses, and long-term health is affected by stage of development at birth (as is now apparent in preterm humans), such species cannot be used to address these questions.
Altered developmental trajectory in twins may be determined in early pregnancy
The physiological reasons behind the altered fetal development, adverse pregnancy outcomes and long-term consequences for adult health that are associated with twin pregnancies are still poorly understood. The dogma is that reduced size at birth and gestational length in twins are related to an inability of the mother to adequately support more than one fetus to term: the competition between the twins for nutrients results in nutrient demand outstripping supply, and there is excessive stretch caused by two fetuses. These two factors then result in initiation of mechanisms that lead to preterm parturition. However, the fetus is known to be a highly effective nutritional parasite, and the signal transduction mechanisms initiated by uterine stretch do not appear to be advanced in multiple pregnancies. In fact, there is no evidence that twins are born earlier than singletons as a result of increased uterine stretch. Data from pregnancies in which the number of fetuses was reduced early in gestation (either spontaneously or through intervention) show a significant association between both gestation length and birth weight and the original number of fetuses, rather than the number that are actually delivered, raising the intriguing possibility that factors in early pregnancy, perhaps even around the time of conception, determine, at least in part, fetal growth trajectory and gestation length in twins.
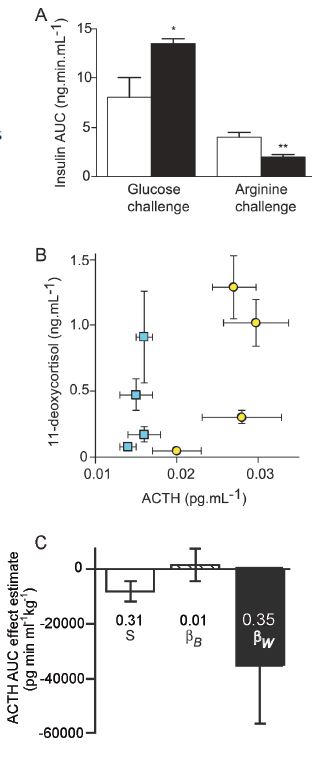
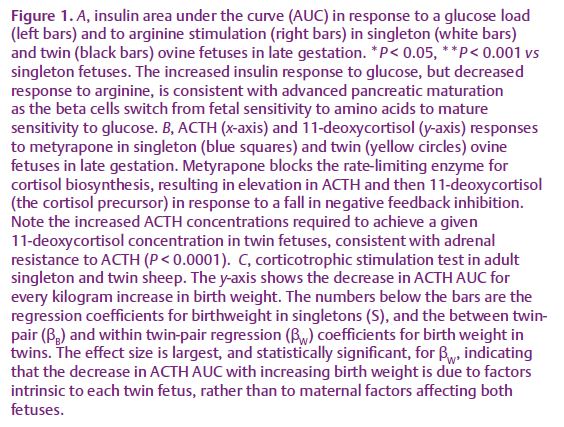
There is good evidence that periconceptional factors affect fetal development in animal studies. For example, we have demonstrated that moderate maternal undernutrition around the time of conception in singleton-bearing ewes (a principally monotocous species) leads to preterm birth and altered fetal development (Bloomfield et al. 2003). Maturation of the fetal hypothalamic pituitary adrenal axis (HPAA) and of the pancreas are advanced. In twin fetuses of well-nourished ewes, pancreatic development is also advanced compared with singletons (Fig. 1A; Rumball et al. 2008). However, other aspects of fetal development in twin fetuses, such as the fetal HPAA, are delayed compared with singletons (Fig. 1B). Ontological studies of HPAA maturation in sheep demonstrate, however, that both the accelerated development in singletons from undernourished ewes and the delayed development in twins are already present in mid-gestation and that placental glucocorticoid metabolism is also altered at this time. Intriguingly, in adult sheep born to periconceptionally undernourished ewes HPAA function is suppressed, whereas in adult twin sheep it is activated, and the degree of activation is related to the within-twin pair coefficient for birth weight (Fig. 1C). Thus, two different periconceptional events both have profound, but different, influences on fetal development that persist as altered physiology in adult life.
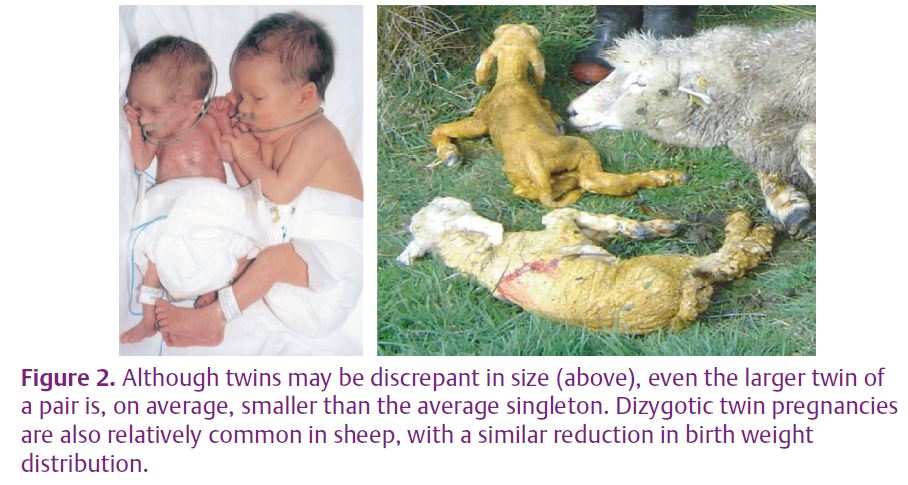
These experimental data are supported by increasing circumstantial evidence that gestation length and size at birth in human pregnancies are also associated with factors operating in the periconceptional period. For example, preterm birth in humans is associated with maternal undernutrition in early pregnancy (Rayco-Solon et al. 2005) and with exposure of the mother to severe stress such as an earthquake. Human studies also suggest that a discrepancy between actual fetal size and expected fetal size in the first trimester predicts both small-for-gestational age and preterm birth (Smith, 2004). Male fetuses are already larger than female fetuses in early human pregnancy, and some twin studies have suggested that growth trajectories of twins diverge from those of singletons as early as 8 weeks gestation.
Do the periconceptional origins of the long-term consequences of being a twin matter?
The periconceptional period appears to be one critical period during which pregnancy outcome can be influenced. Adverse maternal exposures, such as undernutrition, and intrinsic fetal events, such as twin conception, both permanently alter the fetal developmental trajectory with consequences for post-natal physiology. The mechanisms mediating these effects are not yet known, but accumulating evidence suggests that epigenetic modification of DNA and histones may play a role and that epigenomic differences between members of monozygotic twin pairs may explain first trimester predicts both small-for-gestational age and preterm birth (Smith, 2004). Male fetuses are already larger than female fetuses in early human pregnancy, and some twin studies have suggested that growth trajectories of twins diverge from those of singletons as early as 8 weeks gestation.
Do the periconceptional origins of the long-term consequences of being a twin matter?
The periconceptional period appears to be one critical period during which pregnancy outcome can be influenced. Adverse maternal exposures, such as undernutrition, and intrinsic fetal events, such as twin conception, both permanently alter the fetal developmental trajectory with consequences
for post-natal physiology. The mechanisms mediating these effects are not yet known, but accumulating evidence suggests that epigenetic modification of DNA and histones may play a role and that epigenomic differences between members of monozygotic twin pairs may explain phenotypic differences (Poulsen et al. 2007).
The life-long consequences of periconceptional factors, whether they be the nutritional status of the mother or the presence of a co-twin, have implications for both research and for potential interventions. A conundrum for research design is whether fetuses from multitocous species are immune to developmental adaptations in response to additional (or fewer) fetuses, or whether adaptations similar to those demonstrated in monotocous species occur. Clearly, the answer to this question may impact on how findings in animal experiments may translate to the human situation. Further research into understanding the mechanisms and key signalling pathways that mediate signals from the periconceptional environment to the fetus, resulting in setting of developmental trajectory, is critical. Such research may lead to knowledge that could result in interventions before or during very early pregnancy which improve pregnancy outcome.
References
Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JRG & Harding JE (2003). A periconceptional nutritional origin for noninfectious preterm birth. Science 300, 606.
Morse SB, Zheng H, Tang Y & Roth J (2009). Early school-age outcomes of late preterm infants. Pediatrics 123, e622–e629.
Poulsen P, Esteller M, Vaag A & Fraga MF (2007). The epigenetic basis of twin discordance in age-related diseases. Pediatr Res 61, 38R–42R.
Rayco-Solon P, Fulford AJ & Prentice AM (2005). Maternal preconceptional weight and gestational length. Am J Obstetr Gynecol 192, 1133–1136.
Rumball CWH, Harding JE, Oliver MH & Bloomfield FH (2008). Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose–insulin axis function in ovine pregnancy. J Physiol 586, 1399–1411.
Smith GC (2004). First trimester origins of fetal growth impairment. Semin Perinatol 28, 41–50.