
Physiology News Magazine
The placebo response: how words and rituals change brain circuitry
The study of the placebo response is basically the study of the effects of the psychosocial context and the ritual of the therapeutic act on the patient’s brain. Recent research in Parkinsonian patients indicates that the therapist’s words and rituals may induce molecular and cellular changes in the patient’s brain, thus placing the doctor–patient relationship and psychotherapy into the neurobiological domain
Features
The placebo response: how words and rituals change brain circuitry
The study of the placebo response is basically the study of the effects of the psychosocial context and the ritual of the therapeutic act on the patient’s brain. Recent research in Parkinsonian patients indicates that the therapist’s words and rituals may induce molecular and cellular changes in the patient’s brain, thus placing the doctor–patient relationship and psychotherapy into the neurobiological domain
Features
Fabrizio Benedetti (1,2), Michele Lanotte (1) and Leonardo Lopiano (1)
1: Department of Neuroscience, University of Turin Medical School
2: National Institute of Neuroscience, Corso Raffaello 30, 10125 Turin, Italy
https://doi.org/10.36866/pn.77.30

The placebo response, or placebo effect, is the therapeutic effect that follows the administration of an inert treatment (the placebo), for example, a saline solution or a sugar pill. Neither salt nor sugar will ever acquire therapeutic properties. What matters is the psychosocial context around the treatment. In fact, a patient who receives a therapy is deluged with social and sensory stimuli which tell him that a therapy is being performed. This induces expectations of clinical improvement and may affect several systems and apparatuses, producing a real benefit. Therefore, the study of the placebo response is the study of how the patient’s brain is affected by the psychosocial context around the treatment.
Placebos induce dopamine release in Parkinsonian patients
Many medical conditions are affected by placebo administration. Typically, when one administers a placebo, the patient is given a fake treatment along with verbal suggestions of benefit. Overall, the whole ritual of therapy administration constitutes the therapeutic act. There is now compelling experimental evidence that the therapeutic act, together with the doctor’s words and rituals (crucial elements of a placebo procedure), may induce changes in the patient’s brain, such as the release of endogenous opioids and the activation of different brain regions in subjects who are in pain. Likewise, placebos may activate different regions in depressed patients, and may also induce the release of immune mediators and hormones (Benedetti, 2008; Zubieta & Stohler, 2009).
One of the most interesting models and medical conditions that has been investigated to understand the neurobiological underpinnings of the placebo response is Parkinson’s disease, a motor disorder that is highly responsive to placebo treatments. In 2001, de la Fuente-Fernandez et al. (2001) conducted the first brain imaging study of the placebo effect by means of positron emission tomography. These researchers assessed the release of endogenous dopamine by using raclopride, a radiotracer which binds to dopamine D2 and D3 receptors and competes with endogenous dopamine. In this study, patients were aware that they would be receiving an injection of either active drug (apomorphine, a dopamine receptor agonist) or placebo, according to classical clinical trial methodology. After placebo administration, it was found that dopamine was released in the striatum, corresponding to a change of 200% or more in extracellular dopamine concentration and comparable to the response to amphetamine in subjects with an intact dopamine system. The release of dopamine in the motor striatum (putamen and dorsal caudate) was greater in those patients who reported clinical improvement.
Although in the studies by de la Fuente-Fernandez et al. (2001) all patients showed dopamine placebo responses, only half of the patients reported motor improvement. These patients also released larger amounts of dopamine in the dorsal motor striatum, suggesting a relationship between the amount of dorsal striatal dopamine release and clinical benefit. This relationship was not present in the ventral striatum, in which all patients showed increased dopamine release, irrespective of whether they perceived any improvement. Compared to the dorsal motor striatum, the ventral striatum (nucleus accumbens) is involved in motivation and reward anticipation. Accordingly, the investigators proposed that the dopamine released in the ventral striatum was associated with the patients’ expectation of improvement in their symptoms, which could in turn be considered a form of reward.
A placebo treatment changes neuronal activity in the basal ganglia
In 2004, the first study of the placebo effect at the single-neuron level was performed (Benedetti et al. 2004). Since the subthalamic nucleus plays an essential role in basal ganglia functioning and is a major target in the surgical therapy of Parkinson’s disease, we performed a double-blind study in which the activity from single neurons in the subthalamic nucleus before and after placebo administration was recorded to see whether neuronal changes were associated to the clinical placebo response. Before placebo administration, the activity of neurons was recorded from one subthalamic nucleus prior to implantation of the first electrode and used as a control. After the placebo, which consisted of a subcutaneous injection of saline solution along with the verbal suggestion of motor improvement, neuronal activity was recorded from neurons prior to implantation of the second electrode into the other subthalamic nucleus. Those patients who showed a straightforward clinical placebo response, assessed by means of arm rigidity and subjective report of well-being, also showed a significant decrease of firing rate and a shift from bursting to non-bursting activity compared to the pre-placebo subthalamic nucleus. In order to rule out the possibility that the difference in firing rate between the pre- and post-placebo subthalamic nucleus was independent of the placebo treatment itself, a no-treatment group (natural history) was studied. The patients of this no-treatment group did not undergo any placebo treatment between the implantation of the first and second electrode. All these patients showed no significant differences between the neuronal firing rates of the two subthalamic nuclei, which indicates that the difference between the fi rst and the second side of implantation in the placebo group was due to the placebo intervention per se.
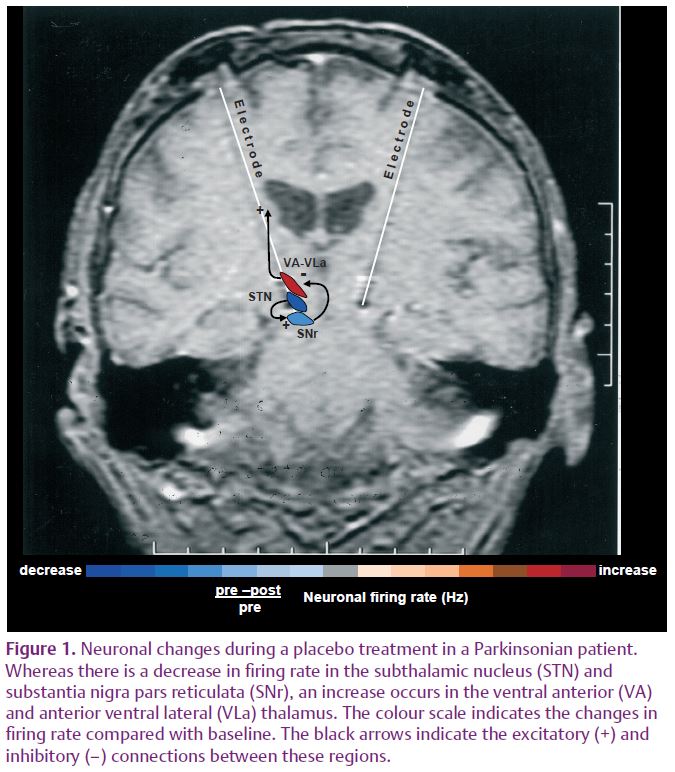
In a more recent study, these findings were extended from the subthalamic nucleus to other nuclei, thereby characterizing a complex neuronal circuit during the placebo response (Benedetti et al. 2009). In those patients who showed a clinical placebo response, there was a decrease in fi ring rate in subthalamic nucleus neurons that was associated with a decrease in the substantia nigra pars reticulata and an increase in the ventral anterior and anterior ventral lateral thalamus (Fig. 1). By contrast, placebo non-responders showed either a lack of changes in this circuit or partial changes in the subthalamic nucleus only. Thus, changes in activity in the whole basal ganglia–thalamic circuit appear to be important in order to observe a clinical placebo improvement. These fi ndings indicate that a placebo treatment, which is basically characterized by verbal suggestions of benefit, can reverse the malfunction of a complex neuronal circuit, although these placebo-associated neuronal changes are short-lasting and occur only in some patients but not in others.
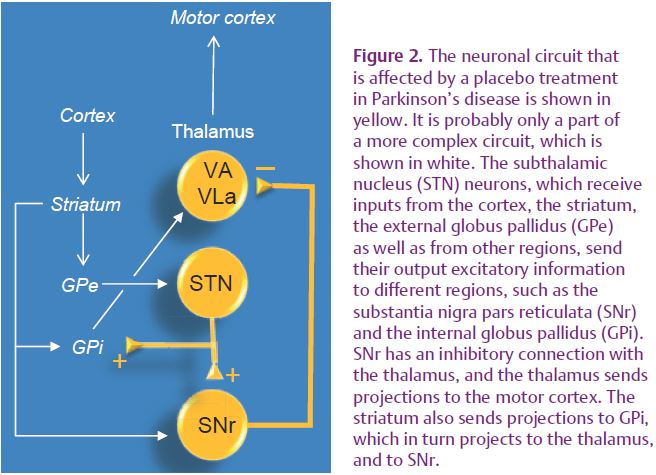
The circuit we have characterized (Benedetti et al. 2009) is likely to be a part of a more complex circuitry, including the striatum and the internal globus pallidus (GPi), that is modified by placebo administration (Fig. 2). These placebo-induced changes may have profound implications for both the doctor–patient relationship and psychotherapy. In fact, the psychosocial context and the therapeutic act itself, along with verbally induced expectations of clinical benefit, may indeed induce specific changes in the patient’s brain, thus placing the therapist–patient communication and psychotherapy into a therapeutic context which per se is capable of modifying the patient’s brain.
References
Benedetti F (2008). Placebo effects: understanding the mechanisms in health and disease. Oxford University Press.
Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B & Lopiano L (2004). Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci 7, 587–588.
Benedetti F, Lanotte M, Colloca L, Ducati A, Zibetti M & Lopiano L (2009). Electrophysiological properties of thalamic, subthalamic and nigral neurons during the anti-parkinsonian placebo response. J Physiol 587, 3869–3883. http://jp.physoc.org/content/587/15/3869. long
de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB & Stoessl AJ (2001). Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 293, 1164–1166.
Zubieta JK & Stohler CS (2009). Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 1156, 198–210.
