
Physiology News Magazine
The sympathetic nervous system and control of resting blood flow in adults with metabolic syndrome
The health consequences of obesity are well known: high blood pressure, high blood glucose and high cholesterol. But alongside these, other sinister effects may be going unmeasured. Does obesity affect the autonomic nervous system? And might a better understanding help us to manage health outcomes?
Features
The sympathetic nervous system and control of resting blood flow in adults with metabolic syndrome
The health consequences of obesity are well known: high blood pressure, high blood glucose and high cholesterol. But alongside these, other sinister effects may be going unmeasured. Does obesity affect the autonomic nervous system? And might a better understanding help us to manage health outcomes?
Features
Jacqueline Limberg, Barbara Morgan & William Schrage
University of Wisconsin, USA
https://doi.org/10.36866/pn.93.36
Obesity epidemic
Over 35% of American adults are obese (Odgden, 2012). Obesity and obesity-related disorders, such as metabolic syndrome, are linked with increased cardiovascular disease risk, the development of type 2 diabetes, and increased all-cause mortality (Carroll & Dudfield, 2004; Ford et al. 2008). Thus, it is not surprising the American Medical Association recently adopted a policy recognizing obesity as a disease (AMA, 2013).
Metabolic syndrome is a condition in which adults are obese, have moderate-to-high blood pressure, high blood glucose, and high cholesterol levels (Ford et al. 2008). Each of these risk factors alone can contribute to adverse health outcomes; however, the clustering of risk factors within an individual increases a person’s risk of developing cardiovascular disease above and beyond each individual factor (Carroll & Dudfield, 2004). In addition to measurable clinical markers (i.e. cholesterol, blood pressure, blood glucose), there are likely to be a number of subclinical adaptations that occur in adults with metabolic syndrome (Carroll & Dudfield, 2004). These potential markers of disease, by definition, go unmeasured during standard medical testing yet are likely to contribute to development of cardiovascular disease and type 2 diabetes in adults with metabolic syndrome. One possible subclinical change includes increased activity of the sympathetic nervous system. Consistent with this idea, elevated sympathetic nerve activity (SNA) has been linked to increased rates of cardiovascular morbidity and mortality and SNA is known to be increased in adults with metabolic syndrome (Lambert et al. 2010).
The sympathetic nervous system
The autonomic nervous system is divided into parasympathetic and sympathetic branches. The sympathetic nervous system, often described as the ‘fight or flight’ nervous system, plays an important role in the body’s response to stress. For example, the anticipatory response to exercise (seen as a rise in heart rate, blood pressure and skin blood flow) is a result of changes in autonomic nervous system activity, including a rise in activity of the sympathetic nervous system.
Activity of the sympathetic nervous system can be measured in a variety of ways, with each method having its own inherent limitations (Mitchell & Victor, 1996; Wallin & Charkoudian, 2007). Microneurography, a technique first described in the late 1960s (Vallbo et al. 2004), allows researchers to safely record sympathetic nerve impulses in awake humans by placing a small, sterile wire microelectrode through the skin into a peripheral nerve carrying sympathetic nerve fibres. Microneurography allows for real-time and direct measurement of SNA headed toward blood vessels in skin and skeletal muscle. Activity of the sympathetic nervous system is identified in the form of sympathetic ‘bursts’ of action potentials (Fig. 1). An increase in the number and/or size of the sympathetic bursts in an SNA recording is associated with increased catecholamine (noradrenaline) release from sympathetic nerve terminals (Wallin et al. 1992, 1996).

Our research primarily focuses on the control of blood flow and blood pressure during physiological stressors such as exercise. Because blood vessels within the skeletal muscle play an important role in determining total peripheral resistance and therefore blood pressure, we will focus our discussion on SNA directed to the skeletal muscle vasculature (muscle SNA, MSNA) and how changes in MSNA regulate blood flow and blood pressure.
Figure 1 shows how an acute increase in MSNA can reduce blood vessel diameter (vasoconstriction) and decrease the amount of blood flow delivered to skeletal muscle. In Step 1 MSNA triggers neurotransmitter release from the sympathetic nerve terminals (e.g. noradrenaline, ATP, neuropeptide Y). In Step 2 neurotransmitters bind to their respective receptors (e.g. adrenergic, purinergic, etc.) on the vascular smooth muscle. In Step 3 receptor binding initiates intracellular signalling that results in vascular smooth muscle contraction and vasoconstriction.
Over time, sustained elevations in MSNA can alter vascular responsiveness to neurotransmitters and cause structural changes in the blood vessels. If these changes cannot be offset by local vasodilatory factors, elevated MSNA may result in a sustained reduction in local blood flow and a rise in arterial blood pressure.
Transient increases in MSNA occur in response to physiological stressors such as mental stress, large-muscle mass exercises, hypotension (low blood pressure), and hypoxia (low oxygen levels). Sustained increases in MSNA are seen with hypertension, obesity, metabolic syndrome, heart failure and sleep apnoea, and are strongly associated with increased mortality risk (Wallin & Charkoudian, 2007; Lambert et al. 2010). Chronic sympathoexcitation is a possible therapeutic target for reducing blood pressure and mortality risk in these disease states (Hering et al. 2012; Paton et al. 2013)
Sympathetic control of the circulation: a balancing act
The exact mechanisms behind chronically elevated resting MSNA are unknown; however, chronic increases in basal MSNA occur in response to advancing age, increases in body fat, systemic inflammation, exposure to sustained or intermittent hypoxia (low oxygen), and altered insulin signalling (Scherrer & Sartori, 1997; Rumantir et al. 1999; Esler & Eikelis, 2006; Esler et al. 2006; Smith & Minson, 2012). With this information in mind, it may be surprising young, healthy adults with normal blood pressures exhibit a wide range of resting MSNA levels (e.g. 5–50 bursts min–1) (Sundlof & Wallin, 1978; Narkiewicz et al. 2005; Wallin, 2007). Thus, rather than the amount of sympathetic activity (MSNA), it may be the integrated response to sympathoexcitation that determines how much vasoconstriction occurs, how much blood flow is reduced, how high blood pressure becomes, and ultimately whether or not increased MSNA contributes to cardiovascular disease.
Interestingly, in young healthy adults, chronic high levels of MSNA are not always associated with increased blood pressure and the development of chronic hypertension (Fig. 2A). This is likely to be the result of a number of checks and balances. Specifically, young men exhibit an inverse linear relationship between MSNA and alpha-adrenergic receptor responsiveness (Charkoudian et al. 2006; Hart et al. 2009, 2012). This means that the blood vessels in young, healthy adults with high basal levels of MSNA do not constrict as much to a given sympathetic ‘signal’ (e.g. MSNA, noradrenaline) when compared to healthy adults with low levels of MSNA (Fig. 2B). This inverse linear relationship may result from adrenergic receptor desensitization and/or downregulation (Steps 2 and 3, Fig. 1) in response to chronic increases in MSNA. Relative to everyday life, this phenomenon might be compared to olfactory fatigue – where prolonged exposure to a particular smell can lead to a temporary inability to perceive the smell.
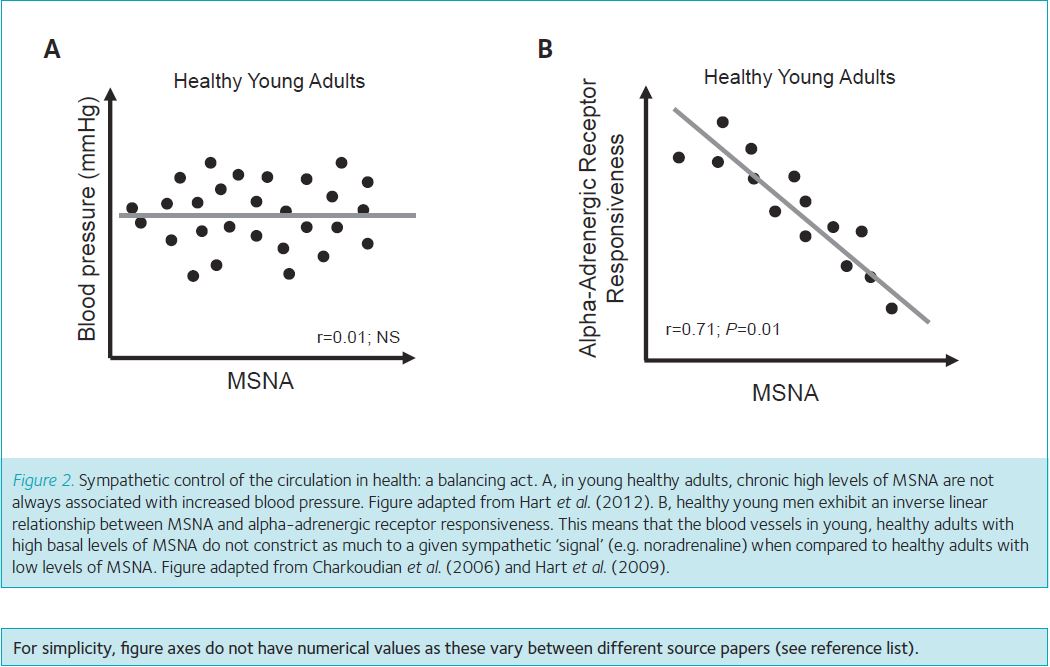
Sympathetic control of the circulation in disease
Obesity-related disorders such as metabolic syndrome are sometimes referred to as ‘early vascular ageing’ because they tend to exhibit similar vascular outcomes to those seen in older adults, including chronically increased MSNA, vascular stiffness, and increased blood pressure. Although MSNA is known to be increased with age, not all older adults have high blood pressure. This may be due to the fact that, similarly to younger adults, older adults exhibit reduced adrenergic receptor responsiveness at rest (Davy et al. 1998; Dinenno et al. 2002) – indicative of receptor desensitization and/or downregulation.
Although research is limited in humans with metabolic syndrome, animal models of metabolic syndrome (often achieved using models of over-feeding and under-exercising) demonstrate increased sympathetically mediated vasoconstriction (Stepp & Frisbee, 2002; Naik et al. 2008). High MSNA that is not counteracted by reduced alpha-adrenergic receptor-mediated vasoconstriction (i.e. the inverse relationship between MSNA and alpha-adrenergic receptor responsiveness seen in healthy adults; Fig. 2B), may be an important factor in the progression of metabolic syndrome toward cardiovascular disease and type 2 diabetes. More specifically, high MSNA combined with high alpha-adrenergic receptor responsiveness in adults with metabolic syndrome could lead to constricted blood vessels, increased blood pressure, and less blood flow, oxygen and glucose delivery to the skeletal muscles.
Hypothesis, experimental design and results
Previous research from animal models of metabolic syndrome suggest several mechanisms by which blood pressure and blood flow control may be altered. However, potential species differences (rat vs. human) limit our ability to translate research directly from animal models to humans. To address this gap in knowledge, we measured basal MSNA and forearm blood flow in adults with metabolic syndrome and healthy control subjects (Limberg et al. 2012). Based on animal models (Stepp & Frisbee, 2002; Naik et al. 2008), we hypothesized adults with metabolic syndrome would exhibit greater alpha-adrenergic mediated vasoconstriction when compared with healthy adults. In addition, we hypothesized the inverse relationship between alpha-adrenergic responsiveness and MSNA observed in healthy subjects (a sign of adrenergic receptor desensitization and/or downregulation) would be lost in adults with metabolic syndrome. Because age can impact sympathetic control of blood flow and blood pressure (Hart et al. 2012), we studied relatively young (mean age 32 years) healthy and metabolic syndrome adults to focus on metabolic syndrome per se independent of any ageing influences.
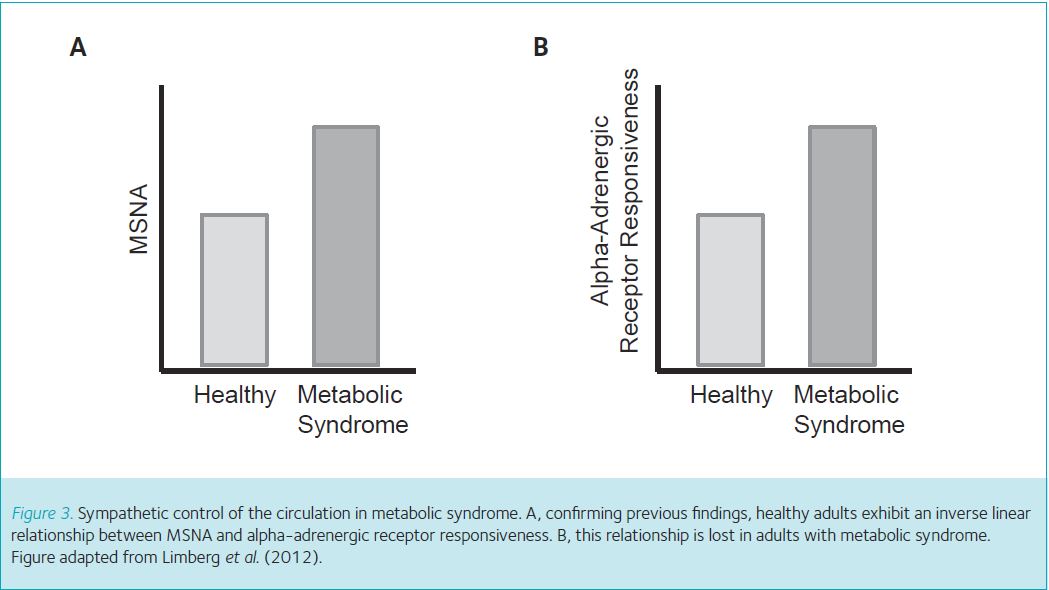
First we used the microneurography technique described above to measure resting levels of MSNA. Confirming previous findings (Lambert et al. 2010), we observed higher levels of MSNA during quiet rest in adults with metabolic syndrome when compared with healthy controls (Fig. 3A). Second, to examine whether alpha-adrenergic receptors in adults with metabolic syndrome were more sensitive to a ‘signal’ to vasoconstrict (Steps 2 and 3), we placed a catheter (flexible plastic tube) into the brachial artery and infused an alpha-adrenergic agonist into the artery (Fig. 1). The selected agonist (phenylephrine) is designed to bind onto alpha-adrenergic receptors on the smooth muscle and cause vasoconstriction, which mimics the action of sympathetic neurotransmitters (Fig. 1, Step 1). This approach allows us to infuse the same concentration of agonist between adults with metabolic syndrome and healthy controls. A greater reduction in blood flow after infusion of phenylephrine indicates greater vasoconstrictor responsiveness. Adults with metabolic syndrome reduced blood flow in the forearm to a greater extent when compared with controls, which indicates adults with metabolic syndrome have greater alpha-adrenergic receptor responsiveness (Fig. 3B). Two key observations were made when we plotted each individual subject’s MSNA (x-axis) and level of alpha-adrenergic responsiveness (y-axis). First, we confirmed previous findings from healthy adults (Figs 2A and 4A). Second, the inverse relationship between alpha-adrenergic vasoconstriction and MSNA is lost in adults with metabolic syndrome (Fig. 4B); this was previously unknown.
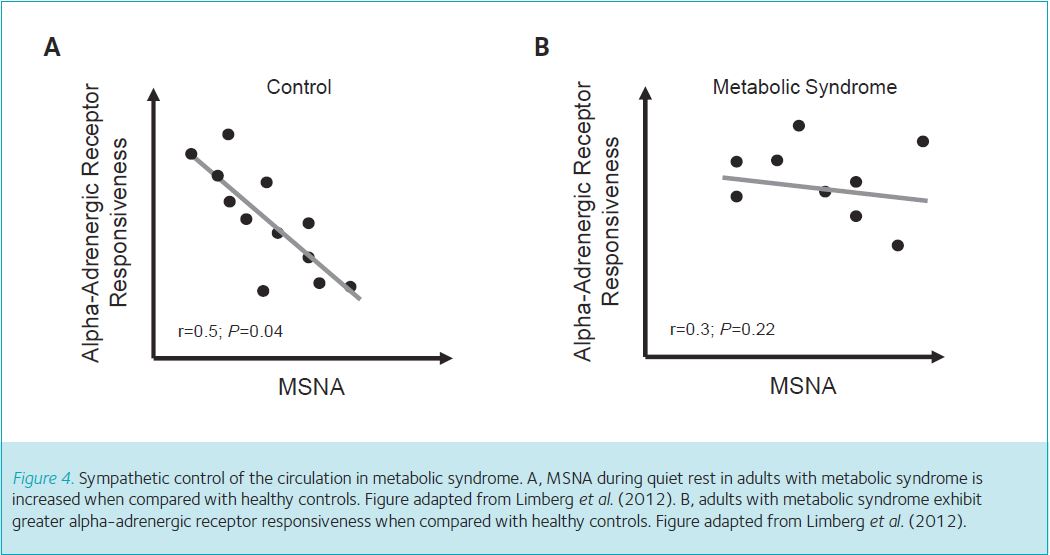
Taken together, MSNA and alpha-adrenergic receptor responsiveness is increased in adults with metabolic syndrome when compared with healthy adults (Limberg et al. 2012). In addition, high levels of MSNA in adults with metabolic syndrome are not counteracted by a reduction in alpha-adrenergic mediated vasoconstriction (Limberg et al. 2012). Thus, unlike young adults, the blood vessels in the resting skeletal muscle of adults with metabolic syndrome do not retain the ability to balance increased MSNA with decreased vasoconstriction. Without this important adaptation, adults with metabolic syndrome are likely to be unable to limit the impact of increased MSNA on systemic vasoconstriction. Consequently, adults with metabolic syndrome may be at a higher risk of impairments in blood flow, glucose disposal to the skeletal muscles, and regulation of blood pressure. Such impairments may promote the progression from metabolic syndrome to type 2 diabetes and overt cardiovascular disease. These results highlight early, pre-clinical changes in relatively young adults with metabolic syndrome that likely contribute to increased cardiovascular disease risk.
The big picture
Over one-third of the population of the United States is obese and at increased risk of developing cardiovascular disease (Odgden, 2012). However, the transition from health to heart failure does not occur overnight. More often, a healthy adult with a normal body composition gradually becomes overweight, then obese, and then develops metabolic syndrome and type 2 diabetes, all prior to the onset of overt cardiovascular disease. Our recent findings (Limberg et al. 2012) add one more clue to understanding the progression from health to disease as it relates to sympathetic control of the circulation. First, alpha-adrenergic mediated vasoconstriction is relatively ‘normal’ in otherwise healthy obese adults (Agapitov et al. 2008). Second, alpha-adrenergic receptors fail to downregulate in a dog model of metabolic syndrome (Dincer et al. 2006). Third, MSNA and alpha-adrenergic responsiveness are increased in human metabolic syndrome (Fig. 3), suggesting alpha-adrenergic receptors fail to downregulate (Fig. 4B). Fourth, alpha-adrenergic mediated vasoconstriction is increased in adults with type 2 diabetes (Hogikyan et al. 1999). Taken together, younger adult humans with metabolic syndrome express an intermediate form of altered sympathetic regulation which may contribute to the development of type 2 diabetes.
Future research
Our study (Limberg et al. 2012) was performed under very controlled conditions; thus there are still many questions to pursue in this research area. First, do all vascular beds adapt the same (e.g. forearm vs. leg vs. heart)? Second, do the sympathetic nerves release the same amount of neurotransmitter (noradrenaline) in adults with metabolic syndrome when compared with healthy adults? Third, do the number of alpha-adrenergic receptors change (e.g. downregulation) as a person develops metabolic syndrome or do signalling pathways within the vascular smooth muscle change? Fourth, are there systemic (e.g. cardiac output) or local (e.g. signals from the vascular endothelium) changes that could help blunt the effects of higher MSNA and possibly delay disease progression in adults with metabolic syndrome? Fifth, is the level of increased MSNA more or less important than the duration (e.g. years) of the disease? Clearly, there are several research questions which remain unanswered and many future directions to be explored.
Conclusion
Considering metabolic syndrome subjects were relatively young and free of overt cardiovascular disease, increased alpha-mediated vasoconstriction may contribute to reduced whole-limb blood flow, altered blood flow distribution, and severe hypertension as the disease progresses. Our recent study combined multiple physiological measures to fill a small, but important gap in our understanding of sympathetic control from health to disease. This approach uncovered some of the earliest subclinical changes in the progression from obesity to metabolic syndrome and type 2 diabetes, and emphasizes the complexity of sympathetic mechanisms of blood flow control.
References
AMA (2013). AMA adopts new policies on second day of voting at annual meeting. http://www.ama-assn. org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page
Agapitov AV, Correia ML, Sinkey CA & Haynes WG (2008). Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension 52, 687–695.
Carroll S & Dudfield M (2004). What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 34, 371–418.
Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB & Wallin BG (2006). Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572, 821–827.
Davy KP, Seals DR & Tanaka H (1998). Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32, 298–304.
Dincer UD, Araiza AG, Knudson JD, Molina PE & Tune JD (2006). Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation 13, 587–595.
Dinenno FA, Dietz NM & Joyner MJ (2002). Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106, 1349–1354.
Esler M & Eikelis N (2006). Is obstructive sleep apnea the cause of sympathetic nervous activation in human obesity? J Appl Physiol 100, 11–12.
Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G & Lambert E (2006). Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48, 787–796.
Ford ES, Li C & Sattar N (2008). Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 31, 1898–1904.
Halliwill JR (2001). Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev 29, 65–70.
Hart EC, Joyner MJ, Wallin BG & Charkoudian N (2012). Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590, 2069–2079.
Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH & Charkoudian N (2009). Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension 54, 127–133.
Hering D, Esler MD & Schlaich MP (2012). Effects of renal denervation on insulin resistance. Expert Rev Cardiovasc Ther 10, 1381–1386.
Hogikyan RV, Galecki AT, Halter JB & Supiano MA (1999). Heightened norepinephrine-mediated vasoconstriction in type 2 diabetes. Metabolism 48, 1536–1541.
Lambert GW, Straznicky NE, Lambert EA, Dixon JB & Schlaich MP (2010). Sympathetic nervous activation in obesity and the metabolic syndrome – causes, consequences and therapeutic implications. Pharmacol Ther 126, 159–172.
Limberg JK, Morgan BJ, Sebranek JJ, Proctor LT, Walker BJ, Eldridge MW & Schrage WG (2012). Altered neurovascular control of the resting circulation in human metabolic syndrome. J Physiol 590, 6109–6119.
Mitchell JH & Victor RG (1996). Neural control of the cardiovascular system: insights from muscle sympathetic nerve recordings in humans. Med Sci Sports Exerc 28, S60–69.
Naik JS, Xiang L, Hodnett BL & Hester RL (2008). Alpha-adrenoceptor-mediated vasoconstriction is not involved in impaired functional vasodilation in the obese Zucker rat. Clin Exp Pharmacol Physiol 35, 611–616.
Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L & Somers VK (2005). Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45, 522–525.
Odgden CL, Carroll MD, Kit BK & Flegal KM (2012). Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief, No. 82. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics.
Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L & Nightingale A (2013). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13.
Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP & Esler MD (1999). Neural mechanisms in human obesity-related hypertension. J Hypertens 17, 1125–1133.
Scherrer U & Sartori C (1997). Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 96, 4104–4113.
Smith MM & Minson CT (2012). Obesity and adipokines: effects on sympathetic overactivity. J Physiol 590, 1787–1801.
Stepp DW & Frisbee JC (2002). Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 282, H816–820.
Sundlof G & Wallin BG (1978). Muscle-nerve sympathetic activity in man. Relationship to blood pressure in resting normo- and hyper-tensive subjects. Clin Sci Mol Med Suppl 4, 387s–389s.
Vallbo AB, Hagbarth KE & Wallin BG (2004). Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol 96, 1262–1269.
Wallin BG (2007). Interindividual differences in muscle sympathetic nerve activity: a key to new insight into cardiovascular regulation? Acta Physiol (Oxford) 190, 265–275.
Wallin BG & Charkoudian N (2007). Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve 36, 595–614.
Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R & Jennings G (1992). Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453, 45–58.
Wallin BG, Thompson JM, Jennings GL & Esler MD (1996). Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491, 881–887.
