
Physiology News Magazine
The trouble with CO2
We breathe for two reasons: to take in O2 and to regulate the amount of dissolved CO2 in the blood. This latter function is fundamental to the control of the pH of blood and hence is a vital homeostatic function. For many years the quest to understand the regulation of breathing by CO2 has concentrated on how the brain measures the pH of blood. New evidence now suggests that the brain also directly measures the level of dissolved CO2.
Features
The trouble with CO2
We breathe for two reasons: to take in O2 and to regulate the amount of dissolved CO2 in the blood. This latter function is fundamental to the control of the pH of blood and hence is a vital homeostatic function. For many years the quest to understand the regulation of breathing by CO2 has concentrated on how the brain measures the pH of blood. New evidence now suggests that the brain also directly measures the level of dissolved CO2.
Features
Nicholas Dale
School of Life Sciences, University of Warwick, Coventry, UK
https://doi.org/10.36866/pn.84.32

The trouble with CO2 is that it readily combines with water to form H2CO3, which dissociates rapidly to H+ and HCO3–. In any solution therefore, the partial pressure of CO2 (PCO2) will be in equilibrium with, and thus inescapably related to, the pH and the concentration of HCO3– of that solution. In terms of physiology, the PCO2 in blood will be an extremely important determinant of the pH of all bodily fluids including the cerebrospinal fluid in the central nervous system. It should come as no surprise that physiological reflexes control the levels of PCO2 via the frequency and depth of breathing, as excretion of CO2 via the lungs is the main mechanism to control its levels in the body. If PCO2 in arterial blood increases, so does the respiratory rate and tidal volume. If the converse happens and PCO2 in arterial blood decreases, then there is a corresponding reduction of respiratory rate and tidal volume.
Any physiological reflex requires at its initiating apex a sensory mechanism – in this case a mechanism of ‘chemosensory transduction’. Here the trouble with CO2 comes to the fore – what does the body measure: CO2, pH, HCO3–, or some combination? All three are inextricably linked and in that sense any one of them would ‘do’. Without knowing the key molecules that are detected by the chemosensors, identifying the molecular transducers involved in the chemosensory reflex is challenging. To make life even more difficult for observers of this field, the phrase ‘CO2 chemosensitivity’ is used loosely to denote responses to changes in PCO2 irrespective of whether this is mediated by direct detection of CO2 or indirectly via consequent changes in pH or HCO3–.
The great German physiologist Loeschcke published an influential review in The Journal of Physiology in 1982, in which he outlined ‘reaction theory’. According to Loeschcke, the respiratory chemosensitive reflexes depended upon the detection of pH and pH alone. This primacy of pH in chemoreception is reflected in other fields of physiology including, for example, CO2 cerebrovascular reactivity and the control of vasodilatation. In the following years pH has been largely regarded as the chemosensory signal and thus much research effort has hinged on studying pH-sensitive ion channels as potential molecular transducers of respiratory chemoreception. So far, genetic manipulation of these channels has failed to provide evidence for their involvement in central mechanisms of chemo reception.
In addition to this identification of pH as the key chemosensory signal, Loeschcke, Schlaefke and Mitchell made the major advance of identifying the ventral surface of the medulla oblongata as being a key site of central CO2 chemosensitivity. The carotid body, of course, provides a peripheral location for CO2 and O2 chemosensitivity, but for CO2 the central sites of chemosensitivity are thought to predominate. Yet even within Loeschcke’s 1982 review, and in his own prior work as well as the subsequent work of other groups (e.g. Eldridge et al. 1985), there are strong indications that pH is not the only chemosensitive signal. For example, comparison of the effect on respiration of applying acidic saline to the medulla oblongata with inhalation of additional CO2 to induce the same pH change in the medulla showed that the combination of an increase in PCO2 and a decrease in pH was a more powerful stimulant than a decrease in pH alone. In retrospect, it seems puzzling that Loeschcke did not acknowledge that these observations were discordant with reaction theory but instead spent considerable effort in ‘arguing them away’. To quote Eldridge et al. (1985): “We conclude that the e.c.f. [extracellular fluid] [H+] does not represent the unique stimulus to the central chemoreceptors. We discuss several alternate mechanisms for the action of CO2 and [H+] on central chemoreceptors but none can be considered definitive at the present time.”
It is also curious that in the intervening years, reaction theory has rarely been challenged and the contradictory evidence has faded from the collective consciousness. Perhaps one reason for this is that it is easy to think about detection of pH in molecular terms – there are many pH-sensitive channels and receptors (e.g. K+ channels of the TASK and inward rectifier gene families and the ASICs (acid-sensing cation channels)). By contrast, CO2-dependent modulation of proteins has remained unfamiliar to physiologists.
However, these interactions are not unknown. Insects such as mosquitos are sensitive to CO2 –thought to be mediated through a G-protein-coupled receptor – but the mechanisms of interaction of CO2 with the receptor remain unknown. The now very old discovery of the effect of CO2 on the affinity of haemoglobin for O2 (the Bohr effect) seems to have been overlooked by physiologists in the sense of what it might mean for the direct detection of CO2 in other contexts. In the Bohr effect, CO2 reacts to form a carbamate moiety (via a rather labile covalent bond) on each of the terminal amines of the two α and two β globin chains. In other fields of biology, notably microbial enzymology and in the fixation of CO2, activation of enzymes by formation of a carbamate moiety on specific lysine side chains is well known. These examples give plausible mechanisms by which CO2 could interact with proteins and thereby be directly detected in physiological systems.
This forms the background to our discovery that connexin 26 (Cx26) – a protein that can form gap junctions – may be a hitherto overlooked receptor for CO2. Connexins are one of two multigene families that can form gap junctions. Gap junctions comprise two hemichannels in each cell that dock together to form a large channel that connects the intracellular contents of such coupled cells. For a long time gap junction hemichannels were rather ignored – seen as being en routeto forming a fully-fledged gap junction. However, hemichannels can indeed have functions of their own – for example, there is well-documented release of ATP through both pannexin and connexin hemichannels. At first little credence was given to physiological roles for hemichannel-mediated ATP release; rather that this was a phenomenon associated with pathology. However, hemichannels can indeed release ATP under normal conditions e.g. in the developing retina and inside taste buds.
Our starting point was the discovery made with collaborators at University College London (Mike Spyer and Alex Gourine) that ATP is released in response to hypercapnia from the chemosensitive areas in the ventral medulla oblongata (the classic areas discovered by Loeschcke and Mitchell). Furthermore, pharmacological data suggested that this ATP release was causally linked to the adaptive respiratory response. Our further analysis of the mechanisms of CO2-evoked ATP release led to the discovery that Cx26 was fundamental to the whole process (Huckstepp et al. 2010a). Firstly, Cx26 is localized in the right place – in specialized glial cells at the ventral surface of the medulla and the membrane (the pia mater) that covers the surface of the medulla. Secondly, ATP release was mediated via a process that was independent of extracellular Ca2+, and could be blocked by hemichannel antagonists, especially those with activity at Cx26. The ATP release depended on PCO2 rather than extracellular pH, and was unaffected by modifiers of intracellular pH. Thirdly, we demonstrated the CO2-dependent loading of carboxyfluorescein into both glial cells and the pia mater at the ventral surface of the medulla – independent evidence that CO2 was able to open channels in these cells large enough to allow ingress of the fluorescent dye, which is otherwise membrane impermeant. Finally, we used the connexin blockers that could antagonize ATP release in response to elevated CO2 to demonstrate that these same blockers, when applied to the ventral surface of the medulla in vivo, blocked both ATP release and the adaptive respiratory response evoked by hypercapnia. Thus, hypercapnia-evoked ATP release at the ventral surface of the medulla in large part appears to be mediated via Cx26 hemichannels.
In a companion paper (Huckstepp et al. 2010b) we analysed the properties of Cx26 themselves. Here again we had a surprise. Although our initial thinking had been that Cx26 was the conduit for ATP release, it turned out that Cx26 had a more fundamental role. Cx26-expressing HeLa cells, unlike their wild type counterparts, are CO2 sensitive – they exhibit CO2-dependent changes in whole cell conductance that have a dose dependence versus PCO2 almost identical to the dose dependence of ATP release from the ventral surface of the medulla; Cx26-expressing HeLa cells can also be loaded with carboxyfluorescein in a CO2-dependent manner; and expression of Cx26 in these cells is sufficient to give them the capacity to release ATP in response to elevated levels of CO2 (the ‘medulla in a cell’). This evidence collectively points to Cx26 being both the CO2 sensor and the conduit for ATP release. Perhaps the strongest evidence for this comes from our demonstration that Cx26 hemichannels still respond to both decreases and increases in PCO2 in excised patches. While we cannot exclude the possibility that there might be an accessory protein necessary for the CO2 sensitivity of Cx26, the simplest interpretation of our data is that Cx26 is indeed directly sensitive to CO2. Interestingly, the closely related connexins Cx30 and Cx32 also exhibit CO2 sensitivity, whereas much more distant connexins (Cx36 and Cx43) are not opened by
increases in PCO2.
Could our discovery of the CO2 sensitivity of Cx26 be the alternate mechanism hypothesized by Eldridge et al. (1985) and quoted above? In the light of our results it seems likely that there are parallel ‘modalities’ of chemoreception – one based on detection of pH and another based on direct detection of CO2 (via Cx26). Although our evidence points to an unexpected locus for chemosensitivity, the pia mater and the astrocytes, a very recent paper from Alex Gourine and collaborators (2010) supports that view and suggests that astrocytes, in addition to being CO2 sensitive, can also respond to pH. This raises the prospect that these parallel modalities of chemoreception may coexist in one class of cell type (Fig. 1).
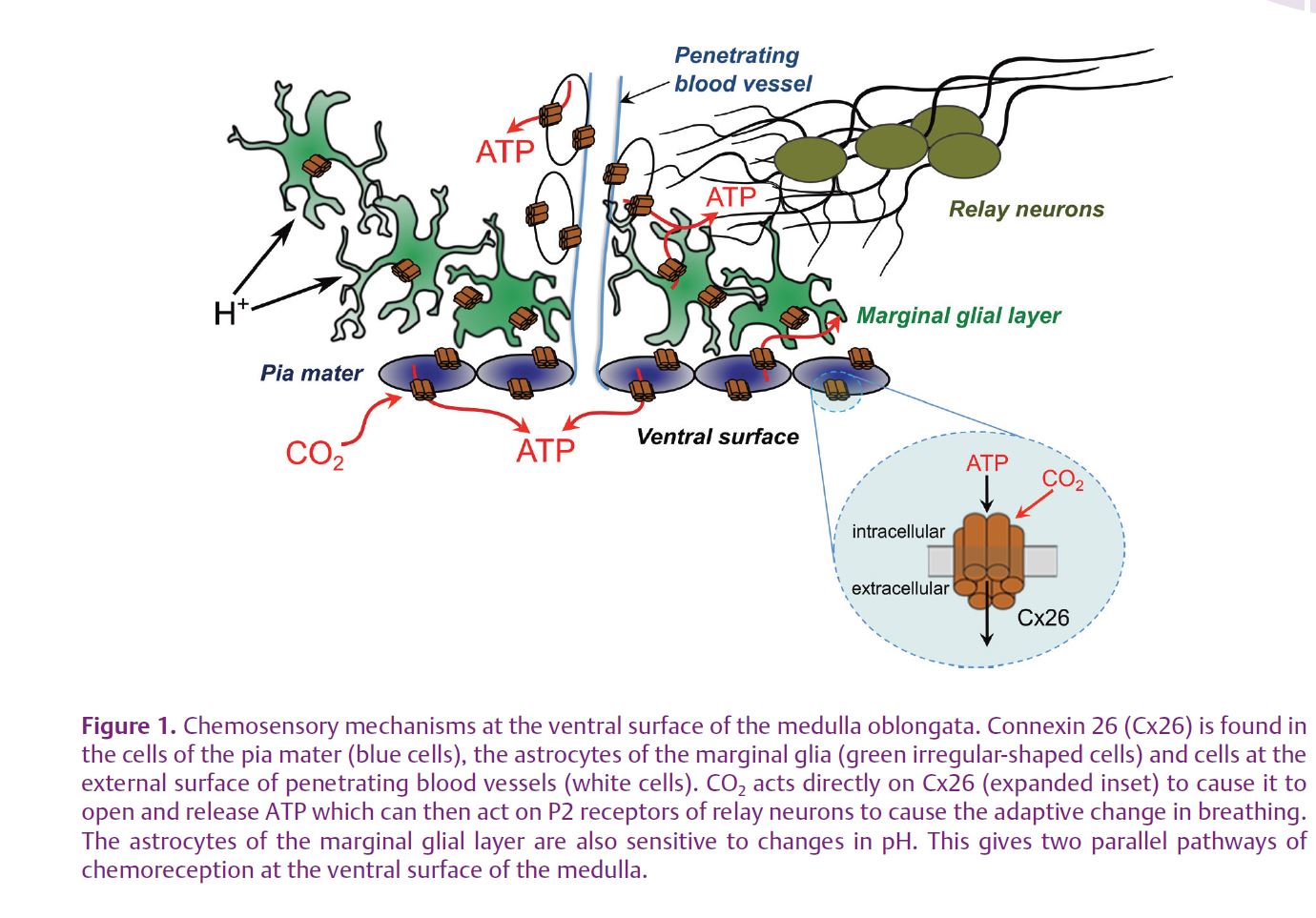
This is an exciting time to be in the field of chemosensory transduction. The recent data showing the importance of astrocytes, the leptomeninges and direct detection of CO2 move the field away from some of its older, more established concepts that assume the primacy of neurons and detection of pH in respiratory chemosensitivity. It will be fascinating to see how prevalent the sensitivity of ion channels to CO2 turns out to be; and how the parallel modalities of pH sensing and CO2 sensing are integrated into complete physiological systems.
References
Eldridge FL, Kiley JP & Millhorn DE (1985). Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol 358, 285–297.
Loeschcke HH (1982). Central chemosensitivity and the reaction theory. J Physiol 332, 1–24.
Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV & Dale N (2010a). Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588, 3901–3920. http://jp.physoc.org/content/588/20/3901.long
Huckstepp RT, Eason R, Sachdev A & Dale N (2010b). CO2-dependent opening of connexin 26 and related β connexins. J Physiol 588, 3921–3931. http://jp.physoc.org/content/588/20/3921.long
Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K & Kasparov S (2010). Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575.
