
Physiology News Magazine
The unique and important role of the myogenic response in the lymphatic system
The lymphatic system provides a critical function, preventing lymphoedema from which 200 million individuals worldwide suffer. Current therapies lack efficacy due to our poor knowledge knowledge of how lymphatic muscle works, including the mysterious role of the myogenic response.
Features
The unique and important role of the myogenic response in the lymphatic system
The lymphatic system provides a critical function, preventing lymphoedema from which 200 million individuals worldwide suffer. Current therapies lack efficacy due to our poor knowledge knowledge of how lymphatic muscle works, including the mysterious role of the myogenic response.
Features
Michael Davis & Joshua Scallan
University of Missouri School of Medicine, Columbia, USA
https://doi.org/10.36866/pn.93.27
The lymphatic system consists of networks of interconnected capillaries, collecting vessels and lymph nodes that absorb, collect and transport the fluid and protein filtered from the blood vascular system (Levick & Michel, 2010). This system provides a critical homeostatic function: in humans, lymphatic vessels return >4 litres of fluid and a substantial amount of protein per day back into the great veins of the neck. Lymphatic vascular dysfunction results in the accumulation of excess fluid (oedema) in the interstitium. Although oedema is typically not life-threatening, it has serious health consequences, including pain, immobility, fibrosis, inflammation, adipose tissue accumulation and tissue damage. Because the lymphatic system is also a critical component of immune responses, lymphoedema is almost always accompanied by an increased risk of infection and other immune system problems (Rockson & Rivera, 2008).
Lymphoedema affects over 200 million people worldwide. Whereas many types of congenital lymphoedema are caused by mutations in genes controlling lymphatic vessel development (Rockson & Rivera, 2008; Alitalo, 2011), the majority of lymphoedema cases in developed nations are acquired secondary to some other type of disease process, such as obesity (Greene et al. 2012; Weitman et al. 2013), congestive heart failure (Witte et al. 1969; Leduc et al. 2011) and peripheral artery or venous disease (Rockson, 1998; Rockson & Rivera, 2008). The largest percentage of cases of acquired lymphoedema, numbering 10–20 million, are patients who develop lymphoedema secondary to breast cancer therapy, recurrent infections, trauma or vascular surgery (Szuba et al. 2003). Symptoms may appear within days or be delayed for years, but ultimately lymphoedema afflicts approximately half of breast cancer survivors who have undergone axillary lymph node dissection (Armer, 2005). There is no cure for lymphoedema and the usual treatment options – massage and/or external compression – only temporarily alleviate symptoms rather than address the underlying cause, which in most instances involves lymphatic tract disruption and/or compromised lymph pumping (Modi et al. 2007; Stanton et al. 2009).
The anatomy of the lymphatic system is key to understanding its function. Lymphatic capillaries interweave among blood capillaries in almost all tissues. The two types of vessels do not normally interconnect; thus lymphatic vessels are nearly invisible due to the absence of lumenal red blood cells (RBCs) (Fig. 1A). Lymphatic capillaries are composed of a single layer of overlapping/interdigitating endothelial cells with discontinuous tight junctions that allow one-way entry of fluid from the tissues. These capillaries eventually coalesce to form collecting lymphatics with a defined basement membrane, at least one muscle cell layer and discrete, one-way intraluminal valves. The muscular collecting lymphatics perform the majority of the work involved in transport through the lymphatic vasculature.
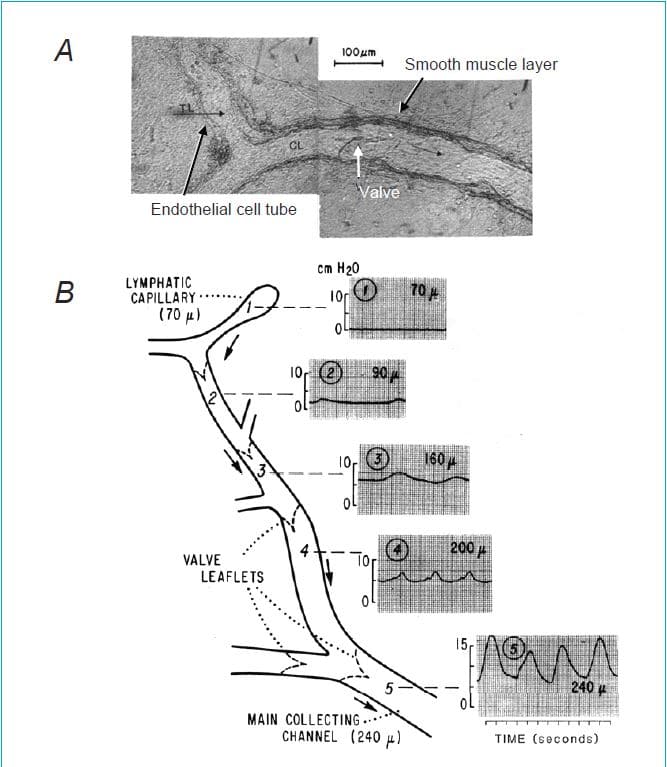
In contrast to the blood vascular system, where the pumping action of the heart generates a pressure head that drives blood ‘downhill’ through arteries, arterioles and capillaries to the venous side of the circulation, the lymphatic system does not rely on this pressure head to move lymph. Instead, in regions of the body below the heart, lymph is propelled ‘uphill’ against a pressure gradient. As illustrated in Fig. 1B, which summarizes one of very few studies measuring the pressure gradient within an intact lymphatic network, the pressure in lymphatic capillaries (or terminal lymphatics (TLs)) is very close to interstitial tissue pressure (~0 cmH2O). Further downstream (i.e. centrally), just before the collecting lymphatics enter a lymph node, the pressure can exceed 15 cmH2O (Zweifach & Prather, 1975). The addition of a hydrostatic column associated with upright posture in a biped would accentuate this gradient. Movement of lymph against this adverse pressure gradient occurs by two mechanisms: (1) the spontaneous, rapid contractions of the smooth muscle cells in the walls of collecting lymphatic vessels, as reflected by the oscillations in intraluminal pressure shown in Fig. 1; or (2) passive compression of the thin-walled lymphatic vessels by external forces, such as might occur with skeletal or visceral muscle contraction. One-way lumenal valves, spaced every few millimetres (in rodents) or centimetres (in larger species) serve the critical function of preventing backflow so that net lymph movement proceeds centrally. Valve opening and closing occur passively in response to the pressure gradient across the valve. The pumping unit, called a lymphangion, is represented by the lymphatic vessel segment bounded by two valves, with one allowing inflow to fill the pump and the other preventing backflow following the ejection of lymph associated with a single pump cycle.
In contrast to common misconceptions, external compressive forces normally account for less than one-third of normal lymph movement in humans, whereas active pumping accounts for over two-thirds (Olszewski, 2002). Indeed, in chronic human lymphoedema, observations of collecting lymphatic vessels in the dependent extremities of patients suggest that the ‘overloading of lymphatics with an excess of continuously produced lymph brings about dilatation of vessels with subsequent insufficiency of unidirectional valves [and] … retrograde flow; the stretched lymphatics … do not generate effective pressures sufficient to propel lymph’ (Olszewski, 2002). It is notable, and unfortunate, that all current therapies for lymphoedema utilize passive compression to promote lymph movement rather than targeting the active lymph pump. This is in large part due to our lack of knowledge of how lymphatic muscle works and how it might be targeted therapeutically.
Work in our laboratory focuses on the role that the muscular, collecting lymphatic vessels play in propelling lymph. To study these vessels under defined conditions, we isolate lymphatic segments from either rat or mouse, cannulate them using micropipettes, and connect them to a two-channel servo system to independently control inflow and outflow pressures. Intraluminal pressure is measured using a servo-null micropipette, internal diameter by edge detection and valve positions by densitometry (see Fig. 2A). We can study segments with a single valve, two valves (a complete lymphangion) or multiple valves (chains of lymphangions). In a series of studies performed over the last 10 years, we have found remarkable similarities between the behaviour of lymphatic muscle and both cardiac muscle (Muthuchamy et al. 2003; Davis et al. 2012; Scallan et al. 2012b; Zhang et al. 2013) and vascular smooth muscle (Gashev et al. 2002; Davis et al. 2009).
The myogenic response of lymphatic vessels is particularly intriguing and will be the focus of the rest of this article. Normally, arterioles respond to an increase in intravascular pressure with constriction and to a decrease in pressure with dilatation; this intrinsic (myogenic) response of arteriolar smooth muscle is likely to aid in protecting capillaries from pressure overload (Davis, 2012). Lymphatic vessels have been known for a long time to react with extreme sensitivity to increases in luminal pressure by increasing contraction frequency (Smith, 1949) and, over a limited pressure range, by increasing contraction amplitude (Zhang et al. 2007).
Our laboratory was the first to show that they also exhibit myogenic constrictions, such that when both inflow and outflow pressures are elevated simultaneously (in the absence of flow), the vessels initially distend, but secondarily develop an increase in basal tone over time (Davis et al. 2009). This response is not only similar in pattern and time course to that of many arterioles, but is comparable in magnitude on a per-unit-pressure basis.
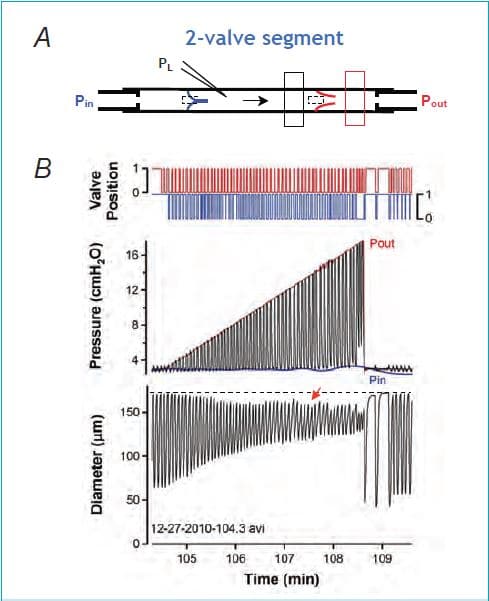
Until recently it has been difficult to envision a homeostatic role for the lymphatic myogenic constriction. In response to pressure elevation, which might occur either as a result of increased lymph formation, partial obstruction of the outflow tract, or elevation in body position, constriction would increase outflow resistance and further retard lymph flow. However, the findings reported in our recent paper in The Journal of Physiology (Scallan et al. 2012a) shed new light on this phenomenon and its potential physiological importance. Instead of elevating both input and output pressures simultaneously as in previous studies, we selectively raised output pressure to a single lymphangion isolated from the rat mesentery, while measuring pressure and diameter in the segment between the two valves (Fig. 2A). Under these conditions the output valve closes and prevents direct transmission of pressure back into the lymphangion. Nevertheless, the vessel responds with a progressive constriction as output pressure is raised in a ramp-wise manner (Fig. 2B). The bottom trace shows the change in diameter, which is alternating during each contraction cycle between a maximum (end-diastolic diameter, EDD) and a minimum (end-systolic diameter, ESD). The middle trace shows the intraluminal pressure change between the two valves: in systole, pressure rises (due to contraction of the muscle layer) to a value that just exceeds the output pressure, Pout, resulting in the opening of the output valve (top trace, red) and ejection; in diastole, pressure falls to a value approximating the input pressure, Pin, allowing the lymphangion to fill from the input pipette when the input valve (bottom trace, blue) opens. A constriction is evident as a decline in EDD (red arrow, compare to dotted reference line). Two factors account for the constriction, the first a myogenic response and the second an increase in contraction frequency. Even though the closed output valve prevents backward transmission of output pressure, a myogenic response occurs because of an increase in time-averaged mean pressure inside the lymphangion (approximately equal to 1/3 of Pout). An increase in frequency dictates that there is less time for filling in diastole, therefore limiting the filling-induced rise in EDD. The vessel shown in Fig. 2B does not show the frequency component because frequency was already elevated at the start of that protocol (the largest change in frequency typically occurs between pressures of 0.5 and 3 cmH2O and that protocol started at 3 cmH2O).
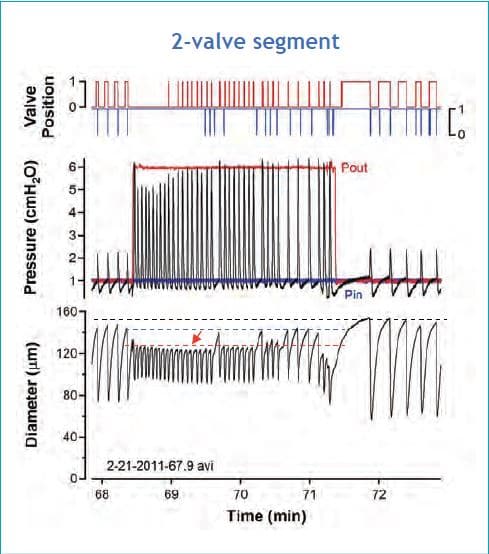
The combination of a myogenic constriction and the effect of a frequency increase induced by output pressure elevation is more clearly evident in vessels starting the protocol at a lower baseline pressure and thus at a lower spontaneous contraction frequency (see Fig. 3). In this example output pressure was elevated step-wise from 1 to 6 cmH2O, to illustrate that a frequency increase can begin during the first contraction cycle following the step. While output pressure remains elevated, frequency remains high, except for six spontaneous pauses that occur; during each of these pauses EDD rises to a value that is intermediate between its initial level prior to Pout elevation and its level during the first cycle after Pout elevation. The component of constriction due to the frequency increase is represented by the difference between the dotted blue and red lines whereas the component due to pressure-induced constriction is represented by the difference between the dotted black and blue lines.
What triggers the increase in contraction frequency in response to elevated output pressure? One-valve segments allow us to separate the myogenic and frequency components of the response because when output pressure is elevated the valve closes and stays closed and pressure on the input side remains at a level near the input pipette pressure (except for very small spikes associated with contractions). The black diameter trace in Fig. 4 shows that a progressive constriction (black arrows) occurs on the input side as Pout is elevated to increasingly higher levels. This constriction is mediated entirely by an increase in frequency, which is initiated on the output side of the valve. The overlaid red diameter trace (offset for clarity) reveals that the output segment actually distends during each Pout step and exhibits a small, secondary myogenic constriction (red arrow, compare EDD to the dotted reference line). The segments on both sides of the valves exhibit synchronized contractions; moreover, the entire vessel segment is synchronized to contract at the highest frequency of any given region. Thus, in response to Pout elevation, the segment on the output side of the valve is distending, increasing its contraction frequency and showing a myogenic constriction (but nevertheless a net dilatation), while pacing the segment on the input side of the valve to also contract at the higher frequency. The input segment undergoes a net constriction because the higher frequency restricts the time for filling during diastole. Subsequently, we used inhibitors, applied selectively to the output side of the segment, to show that the output segment drives the input segment through a signal, probably electrical, that is conducted along the vessel wall across the valve.
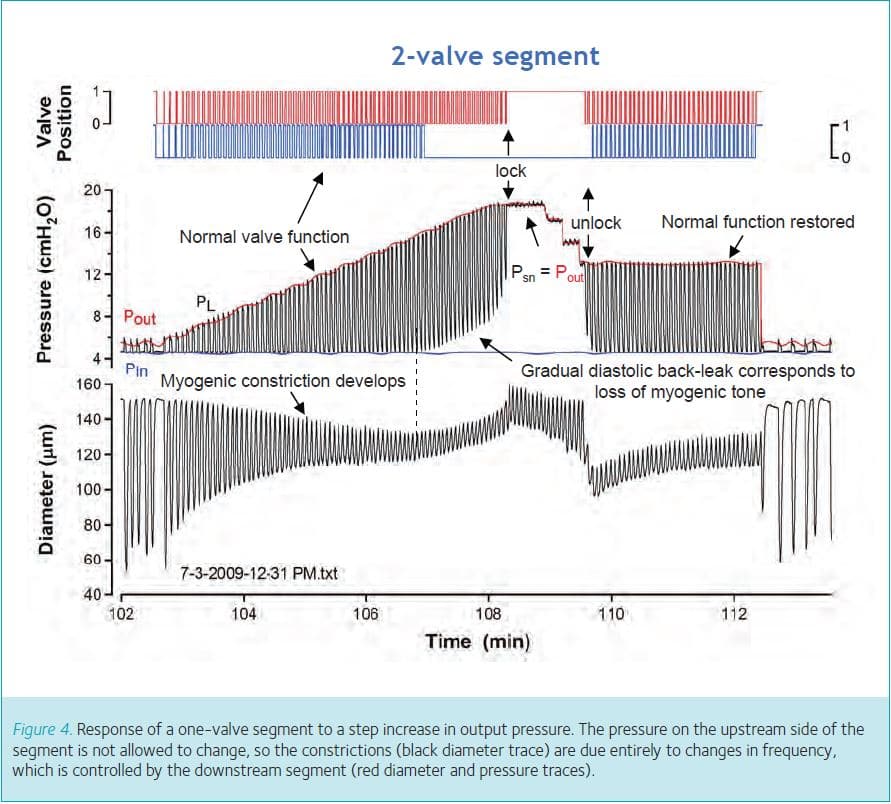
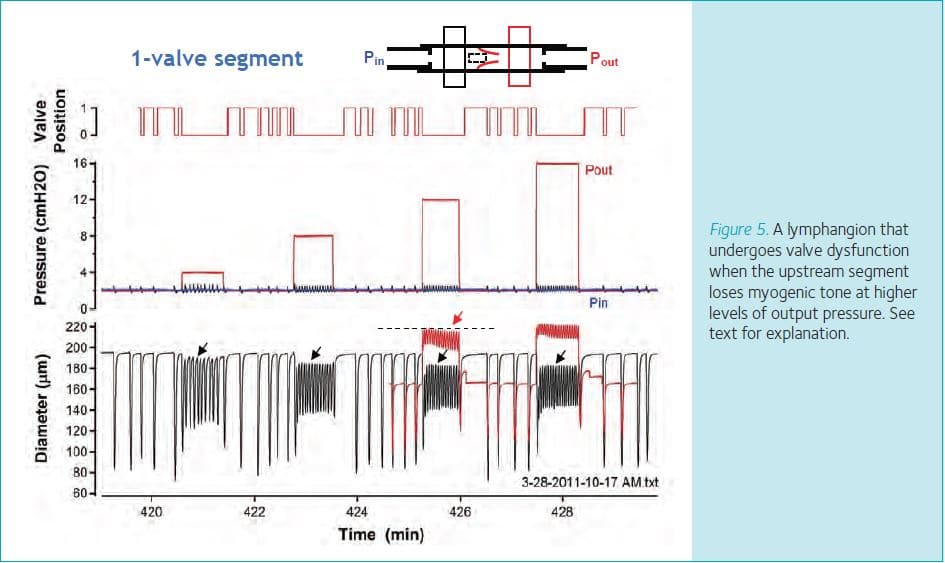
Finally, observations made using two-valve segments provide clues as to why pressure-induced constriction might be physiologically advantageous to the lymphangion. When lymphangions are subjected to increasingly higher levels of output pressure, they show a combined myogenically and frequency-induced constriction initially, but at some point that constriction begins to wane. An example is shown in Fig. 5, where diameter is measured just upstream from the output valve and the constriction wanes at the vertical dotted line. As the input side of the valve loses tone (due to the selective loss of myogenic constriction as frequency remains elevated), the input valve ceases to open (blue valve trace) and diastolic pressure no longer returns to its control value (blue line, equal to Pin), but gradually begins to rise with each subsequent contraction cycle. After about 1 min, pressure equilibrates rapidly across the output valve in the middle of systole, giving the appearance of the valve locking open (‘lock’). In separate studies we demonstrated that when pressures equalize across a valve it reverts to its default position, which is open (Davis et al. 2011). The output valve is not damaged under these conditions because normal function of the valve can be restored by lowering output pressure. However, if output pressure remains elevated this sequence of events has disastrous consequences for the lymphangion because the pump shuts completely down and output pressure is transmitted upstream to the input valve. In chains of lymphangions, we have observed this phenomenon propagate throughout the whole chain until all but one of the valves is ‘locked open’ (unpublished observations). If this sequence leads to transmission of output pressure back to the lymphatic capillaries in vivo, the consequence would be to retard fluid absorption from the interstitium into the lymphatic capillaries and thereby exacerbate oedema formation. Pressure-induced constriction therefore appears to protect the lymphatic valves from becoming overdistended and temporarily insufficient, as well as to protect the lymphatic capillaries from backward pressure transmission.
In conclusion, our studies of single, isolated lymphangions under defined pressure conditions show that collecting lymphatic vessels constrict by a combination of two mechanisms when exposed to elevated output pressure: (1) a pressure-dependent myogenic constriction, and (2) a pressure-induced increase in contraction frequency that leads to a reduction in filling time and decline in diameter. Combined, these two effects produce a stronger constriction (on a per-unit-pressure basis) than myogenic constrictions observed in many arterioles. When pressure is increased at the output end of a lymphatic segment, closure of the closest valve protects the rest of the vessel from the full force of the pressure rise; nevertheless the segment on the upstream side of the valve constricts because the output segment responds with an increase in frequency that drives synchronized contractions of the entire segment at that higher frequency. Although pressure-induced constriction may increase outflow resistance, this deleterious effect on lymph transport may be offset by a need to protect valve regions from becoming overdistended and the valves insufficient.
References
Alitalo K (2011). The lymphatic vasculature in disease. Nat Med 17, 1371–1380.
Armer JM (2005). The problem of post-breast cancer lymphoedema: impact and measurement issues. Cancer Invest 1, 76–83.
Davis MJ (2012). Physiological role(s) of the vascular myogenic response. Microcirculation 19, 99–114.
Davis MJ, Davis AM, Ku CW & Gashev AA (2009). Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296, H293–H302.
Davis MJ, Rahbar E, Gashev AA, Zawieja DC & Moore JE (2011). Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301, H48–H60.
Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA & Zawieja DC (2012). Intrinsic increase in lymphatic muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303, H795–H808.
Gashev AA, Davis MJ & Zawieja DC (2002). Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 450, 1023–1037.
Greene AK, Grant FD & Slavin SA (2012). Lower-exremity lymphoedema and elevated body-mass index. N Engl J Med 366, 2136–2137.
Hargens AR & Zweifach BW (1977). Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233, H57–65.
Leduc O, Crasset V, Leleu C, Baptiste N, Koziel A, Delahaie C, Pastouret F, Wilputte F & Leduc A (2011). Impact of manual lymphatic drainage on hemodynamic parameters in patients with heart failure and lower limb oedema. Lymphology 44, 13–20.
Levick JR & Michel CC (2010). Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87, 198–210.
Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS & Levick JR (2007). Human lymphatic pumping measured in healthy and lympoedematous arms by lymphatic congestion lymphoscintography. J Physiol 583, 271–285.
Muthuchamy M, Gashev A, Boswell N, Dawson N & Zawieja D (2003). Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17, 920–922.
Olszewski WL (2002). Contractility patterns of normal and pathologically changed human lymphatics. Ann NY Acad Sci 979, 52–63.
Rockson SG (1998). Precipitating factors in lymphoedema: myths and realities. Cancer 15, 2814–2816.
Rockson SG & Rivera KK (2008). Estimating the population burden of lymphoedema. Ann NY Acad Sci 1131, 147–154.
Scallan JP, Wolpers JH & Davis MJ (2012a). Constriction of isolated collecting lymphatic vessels in response to acute increases in downsteam pressure. J Physiol 591, 443–449.
Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA & Davis MJ (2012b). Independent and interactive effects of preload and afterload on the lymphatic pump. Am J Physiol Heart Circ Physiol 303, H809–H824.
Smith RO (1949). Lymphatic contractility; a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 90, 497–509.
Stanton AWB, Modi S, Britton TMB, Purushotham AD, Peters AM, Levick JR & Mortimer PS (2009). Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat 117, 549–557.
Szuba A, Shin WS, Strauss HW & Rockson SG (2003). The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphoedema. J Nucl Med 44, 43–57.
Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D & Mehara BJ (2013). Obesity imparis lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8, e70703.
Witte MH, Dumont AE, Clauss RH, Rader B, Levine N & Breed E (1969). Lymph circulation in congestive heart failure: Effect of external thoracic duct drainage. Circulation 39, 723–733.
Zhang R-Z, Gashev AA, Zawieja DC, Lane MM & Davis MJ (2007). Length-dependence of lymphatic phasic contractile activity under isometric and isobaric conditions. Microcirculation 14, 613–625.
Zhang R-Z, Wang W, Gashev AA, Muthuchamy M, Zawieja DC & Davis MJ (2013). Maximum shortening velocity of lymphatic smooth muscle approaches that of striated muscle. Am J Physiol Heart Circ Physiol (in press).
Zweifach BW & Lipowsky HH (1984). Pressure-flow relations in blood and lymph microcirculation. In Handbook of Physiology, section 2, The Cardiovascular System, vol. IV, ed. Renkin EM & Michel CC, Ch. 7, p. 297. American Physiological Society, Bethesda, MD.
Zweifach BW & Prather JW (1975). Micromanipulation of pressure in terminal lymphatics in the rat mesentery. Am J Physiol 228, 1326–1335.
