Physiology News Magazine
Thought to action: development of temporal signals from topographic maps
Features
Thought to action: development of temporal signals from topographic maps
Features
David M Waitzman (1,2) & Jason A Cromer (1,2)
1: Department of Neurology and 2: Program in Neuroscience
University of Connecticut Health Center, Farmington, CT, USA
https://doi.org/10.36866/pn.66.23

Maintaining the stability of the eye during vision and shifting the eye rapidly to view different locations are two of the major tasks of the oculomotor system. Different populations of neurons in the visual pathways become active depending on where light falls on the retina (a spatial code). In contrast, single motor neurons that drive the muscles of the eyes encode the amplitude of muscle contraction via the number of spikes in their burst and the speed of contraction by the rate at which spikes are emitted (a temporal code). To perform the task of repositioning the eye with a high degree of accuracy, the central nervous system must provide a rapid and accurate mechanism via which activity related to a sensory perception is translated into appropriate movements. In neuronal terms, this question can be rephrased into understanding how the activity of topographically arranged, spatially encoded neurons of the visual system is transformed into the time varying spike train of the motor neurons that is necessary to drive the eye muscles. This article summarizes recent data suggesting how the change from spatial to temporal coding occurs.
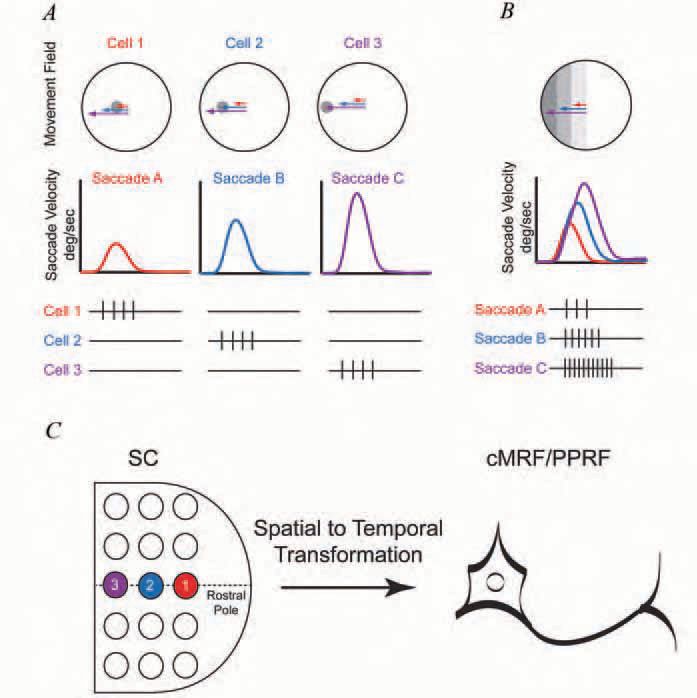
The superficial layers of the superior colliculus (SC) located in the dorsal portion of the midbrain receive direct retinal and visual cortical input and contain a topographic map of the contralateral field of vision comparable to that in the visual cortex (see Wurtz & Goldberg, 1989 for review). Below the superficial layers, neurons in the intermediate and deep layers of the SC are activated just before the occurrence of rapid eye movements that shift the fovea a specific amplitude and direction relative to its previous position. In analogy to the sensory receptive field of the visual system, the array of eye movements which activate a SC neuron describes a ‘movement field’ (Fig. 1A).This structural arrangement of a pure sensory region above and a ‘movement associated layer’ below sparked considerable interest and suggested an opportunity to explore the transformation of visual information into motor activity, a so-called spatial to temporal transformation or STT (Fig. 1C) (Sparks & Mays, 1990).
However, despite an exhaustive evaluation of the SC activity, the underlying nature of this transformation remains elusive. Indeed, most investigators agree that the discharge of neurons in the intermediate and deep layers of the SC encode the intended target of a saccade, but do not encode the metrics of the movement (i.e., neither the number of spikes nor the rate of discharge is related to movement amplitude or velocity) (Bergeron et al. 2003). Thus, one of the major questions in oculomotor control has been to understand how temporal signals like those of the neuron shown in Fig. 1B that encode the dynamics of saccadic eye movements are developed.
Location of temporally coded neurons
Temporal signals encoding eye movement dynamics are the end result of the STT. Over the last three decades a number of laboratories have searched for evidence of such signals in the structures targeted by neurons of the intermediate and deep layers of the SC (see Moschovakis et al. 1996 and Scudder et al. 2002 for reviews). Areas of particular interest are positioned downstream of the SC and include portions of the mesencephalic and pontine reticular formations (MRF and PPRF) and the nucleus reticularis tegmenti pontis (NRTP), the primary gateway to the cerebellum for the oculomotor system. These structures provide either direct or indirect access to abducens motor neurons.
The paramedian portion of the pontine reticular formation (PPRF) forms part of the direct pathway. The PPRF receives anatomically weighted, contralateral projections from the SC (Grantyn et al. 2002) and the axons of its burst neurons impinge directly upon abducens motoneurons (Fig. 1C, Fig. 2). The number of spikes in each burst of a PPRF neuron is monotonically related to the amplitude of each saccade while the discharge frequency of the PPRF burst neurons is directly related to the instantaneous velocity of the saccade (Fig. 1B) . The duration of the PPRF burst is tightly regulated by a group of inhibitory neurons located along the midline of the pons in the raphe interpositus (Buttner-Ennever et al. 1988). Since these neurons have a tonic level of activity and pause for saccades in all directions, they have been dubbed ‘omnipause neurons’ (OPNs) (Fig. 2).

The central Mesencephalic Reticular Formation (cMRF), located just lateral to the oculomotor nuclei and ventral to the superior colliculus (Horn, 2005), receives ipsilateral afferents from the SC and it forms reciprocal connections with the SC and the omnipause neurons The majority of cMRF neurons provide a burst of spikes that are most closely associated with contralateral saccades. Recent recordings from cMRF neurons have demonstrated that the number of spikes and frequency of discharge in the saccade associated bursts are correlated with saccade amplitude and instantaneous velocity similar to the bursts in PPRF neurons (Fig. 1B)
(Cromer & Waitzman, 2006). Indeed, many of the neurons had a monotonically increasing relationship between spike number and saccade amplitude and burst duration was closely correlated with saccade duration. While the correlations for cMRF burst neurons are generally weaker than those in the PPRF, nevertheless, these temporal signals could influence motor neurons via direct projections from the cMRF to the abducens nucleus (Ugolini et al. 2006) or via an indirect path mediated via reciprocal connections with the omnipause neurons (Fig. 2).
The spatial to temporal transformation
Despite the identification of temporally encoded neurons, the question remains as to how a topographically defined locus of activity in the superior colliculus that occurs approximately 40 ms in advance of saccades is molded into a burst of impulses related to saccade amplitude, duration, and instantaneous velocity that can activate extraocular motor neurons 20 to 25 ms later?
While neurons in the SC do not typically display relationships to saccade metrics, their discharge duration may reflect changes that occur in downstream structures (Goossens & Van Opstal, 2000; Soetedjo et al. 2002b). However, the close relationship between the discharge of cMRF and PPRF neurons to instantaneous eye velocity requires a physiological mechanism that can utilize the locus of SC activity, which is poorly associated with the metrics of the saccade, to generate the highly correlated time varying signals related to eye amplitude and velocity (Fig. 1C). While the density of projections between the SC and the PPRF likely contributes to the generation of these temporal signals (Fig. 2), it cannot be the only mechanism via which these tightly controlled signals are created.
A number of physiologic models have been postulated to explain how the STT occurs. One suggestion is that a series of SC neurons projecting onto neurons in the PPRF are sequentially activated at a particular rate to develop not only the correct spike number, but the time variation associated with velocity found in these target neurons (Munoz & Wurtz, 1995). However, physiological evidence for such a spread of activity across the primate SC has not been found (Keller & Edelman, 1994; Soetedjo et al. 2002a). An alternative idea suggests that the SC provides an idealistic drive and that the exact metrics of the pontine burst are shaped by cerebellar outflow that also impinges on the PPRF neurons (Quaia et al. 1999). This model shifts the idea of spreading activity away from the SC to the cerebellar vermis, for which again there is little physiological evidence (Robinson & Fuchs, 2001).
A different approach has posited that a recursive excitation loop between the cMRF and the SC could prolong the collicular outflow (Cromer & Waitzman, 2006). In this schema activation of a collicular locus would trigger excitation in this recursive pathway and possibly a similar loop between the cMRF and the omnipause neurons (Fig. 2). These recursive loops could generate a persistent burst whose number of spikes and duration corresponded with the desired saccade amplitude as long as a second signal appeared to appropriately shut down the loop thereby ending the saccade. The origin of this ‘shut-down’ signal is unclear, but could arise via a second indirect pathway that utilizes projections from the SC to the NRTP which projects in turn to the fastigial oculomotor region of the the cerebellar vermis whose cells are activated with saccade end (Ohtsuka & Noda, 1991; Fuchs et al. 1993; Quaia et al. 1999). While the exact mechanism for the STT remains an area of active research, generation of the burst in PPRF neurons that is tightly coupled to saccade dynamics probably occurs via modulation of the direct tecto-pontine projection (Fig. 2, direct pathway) by indirect pathways. This idea is attractive since it keeps most of the machinery necessary for developing and monitoring the time varying signals for saccades downstream from the SC. Moreover, this approach would permit topographically organized cortical regions such as the frontal eye fields access to the same eye movement generator utilized by the superior colliculus.
References
Bergeron A, Matsuo S & Guitton D (2003). Superior colliculus encodes distance to target, not saccade amplitude, in multi-step gaze shifts. Nat Neurosci 6, 404-413.
Buttner-Ennever JA, Cohen B, Pause M & Fries W (1988). Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol 267, 307-321.
Cromer JA & Waitzman DM (2006). Neurones associated with saccade metrics in the monkey central mesencephalic reticular formation. J Physiol 570, 507-523.
Fuchs AF, Robinson FR & Straube A (1993). Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol 70, 1723-1740.
Goossens HH & Van Opstal AJ (2000). Blink-perturbed saccades in monkey. II. Superior colliculus activity. J Neurophysiol 83, 34303452.
Grantyn A, Brandi AM, Dubayle D, Graf W, Ugolini G, Hadjidimitrakis K & Moschovakis A (2002). Density gradients of trans-synaptically labeled collicular neurons after injections of rabies virus in the lateral rectus muscle of the rhesus monkey. J Comp Neurol 451, 346-361.
Horn AK (2005). The reticular formation. Prog Brain Res 151, 127155.
Keller EL & Edelman JA (1994). Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol 72, 2754-2770.
Moschovakis AK, Scudder CA & Highstein SM (1996). The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol 50, 133-254.
Munoz DP & Wurtz RH (1995). Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J Neurophysiol 73, 2334-2348.
Ohtsuka K & Noda H (1991). Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol 65, 1422-1434.
Quaia C, Lefevre P & Optican LM (1999). Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 82, 999-1018.
Robinson FR & Fuchs AF (2001). The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24, 981-1004.
Scudder CA, Kaneko CS & Fuchs AF (2002). The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142, 439-462.
Soetedjo R, Kaneko CR & Fuchs AF (2002a). Evidence against a moving hill in the superior colliculus during saccadic eye movements in the monkey. J Neurophysiol 87, 2778-2789.
Soetedjo R, Kaneko CR & Fuchs AF (2002b). Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87, 679-695.
Sparks DL & Mays LE (1990). Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci 13, 309336.
Ugolini G, Klam F, Doldan Dans M, Dubayle D, Brandi AM, Buttner-Ennever J & Graf W (2006). Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: Differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol 498, 762-785.
Wurtz RH & Goldberg ME (1989). The Neurobiology of saccadic eye movements. Elsevier, New York, NY.