
Physiology News Magazine
Tissue biomechanics as a modulator of lymphatic function
The lymphatic network represents a good example of how biological structures maximise their efficiency by exploiting the anatomical and biomechanical properties of their microenvironment. Indeed, the stiffness of the extracellular matrix may directly affect the absorptive and/or propulsive nature of a lymphatic vessel, thus exerting an important modulatory role in lymphatic function.
Features
Tissue biomechanics as a modulator of lymphatic function
The lymphatic network represents a good example of how biological structures maximise their efficiency by exploiting the anatomical and biomechanical properties of their microenvironment. Indeed, the stiffness of the extracellular matrix may directly affect the absorptive and/or propulsive nature of a lymphatic vessel, thus exerting an important modulatory role in lymphatic function.
Features
Daniela Negrini and Andrea Moriondo
Department of Experimental and Clinical Biomedical Sciences, University of Insubria, 21100 Varese, Italy
https://doi.org/10.36866/pn.84.30


The diaphragmatic lymphatic network represents a good example not only of the mechanisms of lymph formation and transport along the complex lymphatic microvasculature, but also of how the biological structures maximise their efficiency with minimal expense by exploiting in the best possible way the anatomical and biomechanical properties of their biological microenvironment. In the extracellular compartment, freely moving water is distributed between the intravascular plasma and the interstitial fluid filling the porosity of the three-dimensional extracellular fibrous matrix. Interstitial fluid derives from plasma and is mostly filtered through paracellular pathways between adjacent endothelial cells, down a favourable transendothelial pressure gradient resulting from the imbalance of hydraulic and colloidosmotic pressures. A crucial element of this process is interstitial fluid pressure (Pint), a complex parameter which simultaneously depends upon tissue hydration, the mechanical behaviour of the matrix and the osmotic forces exerted by the matrix itself. At normal tissue hydration, baseline Pint is usually subatmospheric and may change, either towards more positive or more negative values, in response to the displacement of the surrounding tissue, thus affecting the transendothelial fluid filtration process. For example, in the lung parenchyma, characterised by very negative end-expiratory Pint values, fluid filtration occurs across both the arteriolar and venular side of the capillary and further increases when Pint drops on inspiration. Convective fluid filtration from the microvasculature to interstitium is accompanied by solutes and protein fluxes. Therefore, progressive departure of plasma volume and proteins and an increase of interstitial fluid volume (a condition called oedema), would occur if excess interstitial fluid and solutes were not continuously removed into the initial lymphatics, small dead-end saccular structures dispersed among the matrix fibres. The lymphatic system has developed in the majority of body organs of vertebrates to remove fluid, solutes and even cells from the tissues and return them to the venous blood stream, thus maintaining fluid and solute homeostasis in both the extravascular and intravascular environments. Lymph flow is sustained by pressure gradients (ΔPTM-lymph) developing when intraluminar lymphatic pressures drop below Pint; the newly formed lymph is thereafter directed, with the aid of unidirectional intraluminar valves, into larger collecting lymphatics, equipped with contractile smooth muscle cells. Favourable ΔPTM-lymph levels develop as a consequence of cyclic contraction of lymphatic smooth muscle cells and/or of tissue displacement (Schmid-Schöenbein, 1990; Aukland & Reed, 1993). For example, during spontaneous breathing, the average ΔPTM-lymph in intercostal lymphatics over a minute ventilation is positive (~4–10 mmHg) (Moriondo et al. 2005), suggesting that rhythmic expansion of the chest favours lymph formation. However, when the same tidal volume is attained through mechanical ventilation with positive alveolar pressure, ΔPTM-lymph is nullified (Moriondo et al. 2005), indicating that not simply the degree of tissue displacement, but rather the magnitude and direction of local forces developing across the lymphatic vessel wall is critical in supporting lymphatic function.
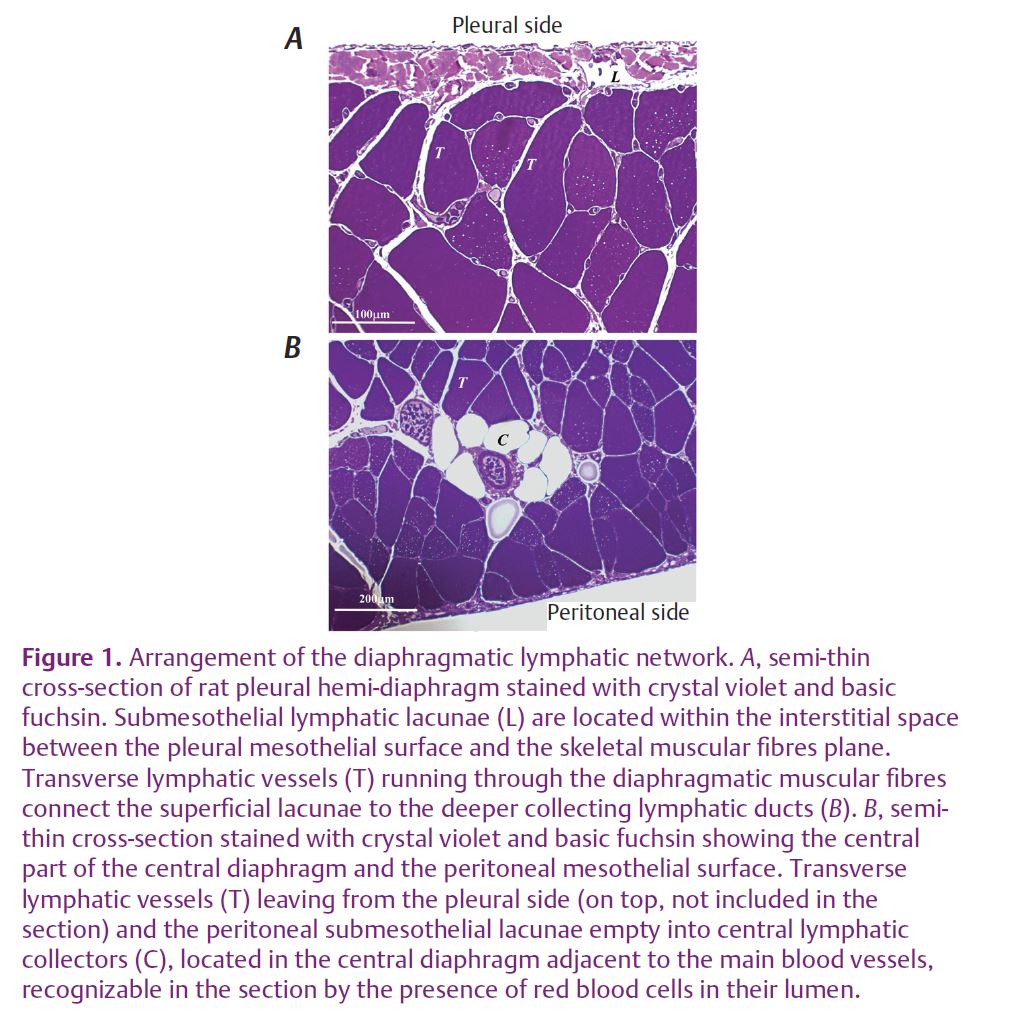
For tissue displacements to be able to support the development of ΔPTM-lymph, thereby promoting lymph formation and further progression along the network, local tissue stresses must be transmitted to the lymphatic lumen and converted into intraluminar pressure waves. In turn, force transmission is influenced by the mechanical properties of the vessel wall and is expected to be more effective in stiff vessels characterized by a lower compliance compared to distensible, highly compliant structures. Even if belonging to the same drainage network, the diaphragmatic lymphatic vessels display a rather remarkable heterogeneity. Indeed, deepening from the pleural and peritoneal mesothelial surfaces into the diaphragmatic tissue, the lymphatic network is organised in: (a) submesothelial lacunae, located above or partially within the submesothelial interstitial space (Figs1A and 2A); (b) deep central collectors partially equipped with smooth muscle cells (Figs 1B, and 2B), transverse lymphatic ducts (Fig.1A and B) running through muscular and tendinous diaphragmatic fibres and connecting the submesothelial lacunae to the central collectors (Grimaldi et al. 2006).
A finite element model of the mechanical properties of the vessels based on direct measurements
of wall compliance in lymphatic vessels running over the pleural diaphragmatic surface and of the elastic modulus of the tendinous and muscular relaxed diaphragmatic tissue (Moriondo et al. 2011) revealed that these vessels exhibit changed mechanical behaviour in relation to the different structure of their wall and of the surrounding tissue. Vessels delimited by a compliant tissue act as distensible fluid reservoirs, whose walls are subject to high tangential stresses favouring the opening of primary unidirectional valves in the vessel wall (Fig. 2A); these vessels, corresponding in the diaphragm to the submesothelial diaphragmatic lacunae, are therefore favourable sites of drainage of fluid from the pleural and the peritoneal cavities. In contrast, vessels surrounded by stiff tissue, such as those running deep among diaphragmatic muscular or tendinous fibres (Fig. 2B), are characterized by a fast and efficient transmission of tissue forces to the vessel lumen and are therefore better suited to propel the newly formed lymph along the lymphatic collecting network. Therefore, more generally in the lymphatic system, vessels supplying skeletal muscle or stiff actively moving tissues, such as the lung, may not necessarily need the phasic synchronized contraction of lymphatic smooth muscle cells usually encountered in most collecting ducts to sustain local lymphatic function.
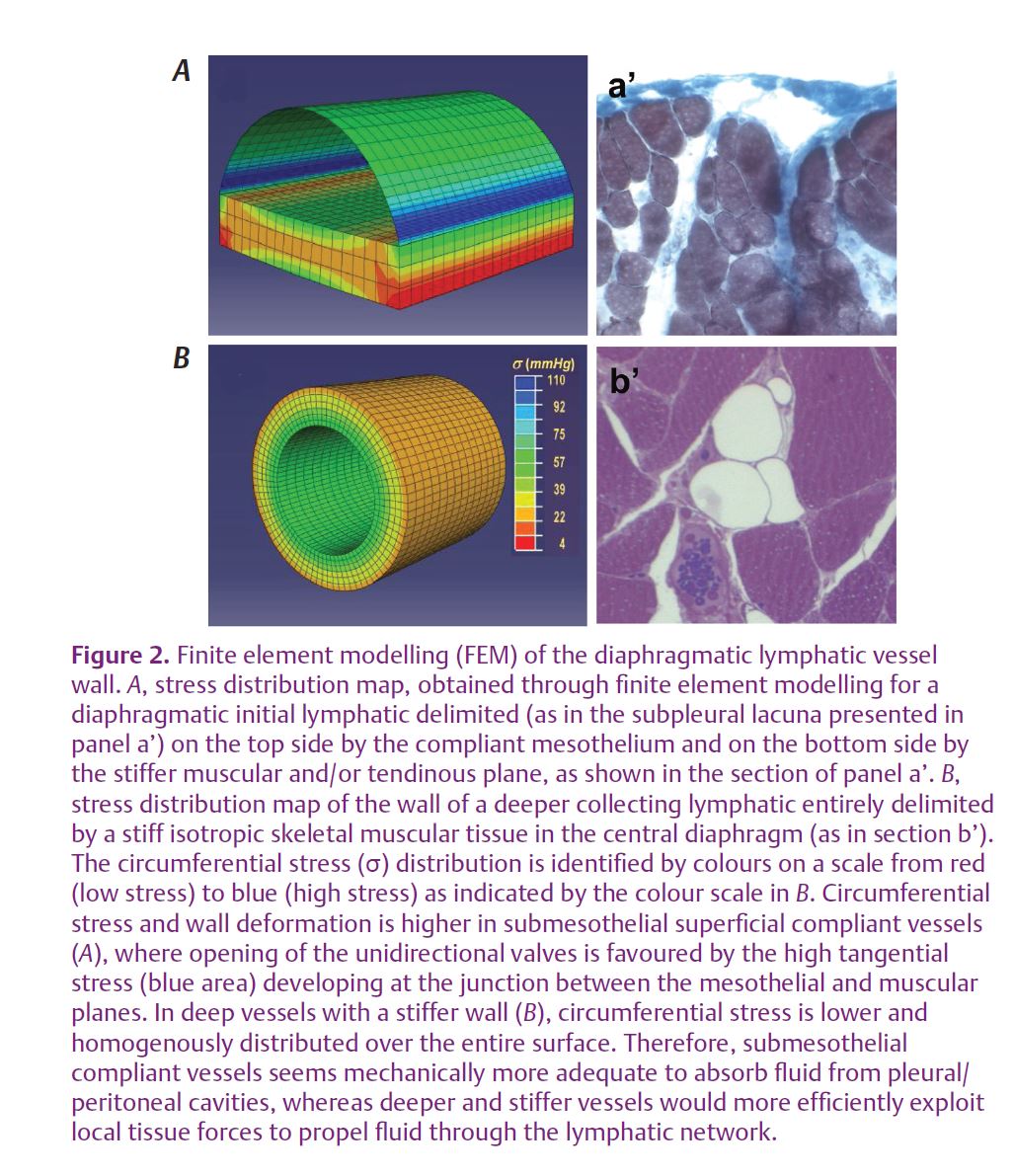
Based on these considerations, the arrangement of the matrix fibres and their mechanical properties emerge as an important factor in setting, maintaining and modulating the local lymph flux in initial lymphatics and therefore in the entire lymphatic network. It is worth recalling that, even in collecting lymphatics, wall stiffness and therefore the mechanical response of the vessels is both phasically and tonically increased by contraction of smooth muscle cells in the external vessel layer. Therefore, a large variety of pathophysiological conditions, such as neuromuscular failure, skeletal motor weakness, disturbance of smooth muscle contraction, disorganization of the macromolecular composition and/or arrangement of the fibrous tissue scaffold, like that observed during development of pulmonary oedema (Negrini et al. 1996), by modifying the mechanical features of the lymphatic vessel wall and adjacent tissues, may greatly impair normal lymphatic function, thus contributing to the development of oedema and exacerbation of tissue and organ damage.
References
Aukland K & Reed R (1993). Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73, 1–78.
Grimaldi A, Moriondo A, Sciacca L, Guidali ML, Tettamanti G & Negrini D (2006). Functional arrangement of rat diaphragmatic initial lymphatic network. Am J Physiol Heart Circ Physiol 291, H876–H885.
Moriondo A, Boschetti F, Bianchin F, Lattanzio S, Marcozzi C & Negrini D (2010). Tissue contribution to the mechanical features of diaphragmatic initial lymphatics. J Physiol 588, 3957–3969. http://jp.physoc.org/content/588/20/3957.long
Moriondo A, Mukenge S & Negrini D (2005). Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol 289, H263–H269.
Negrini D, Passi A, De Luca G & Miserocchi G (1996). Pulmonary interstitial pressure and proteoglycans during development of pulmonary edema. Am J Physiol Heart Circ Physiol 270, H2000–H2007.
Schmid-Schöenbein G (1990). Micro-lymphatics and lymph flow. Physiol Rev 70, 987–1019.
